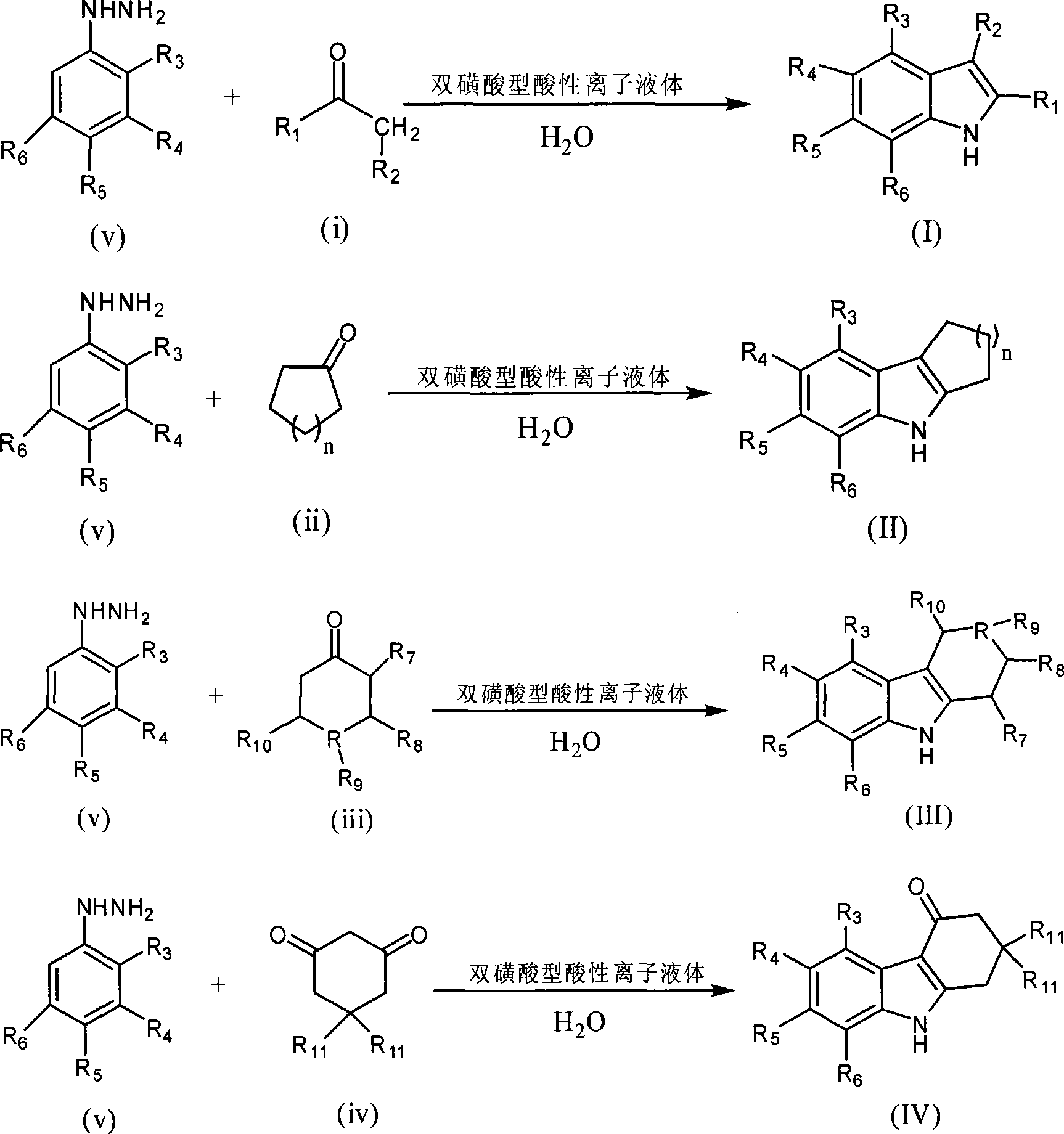
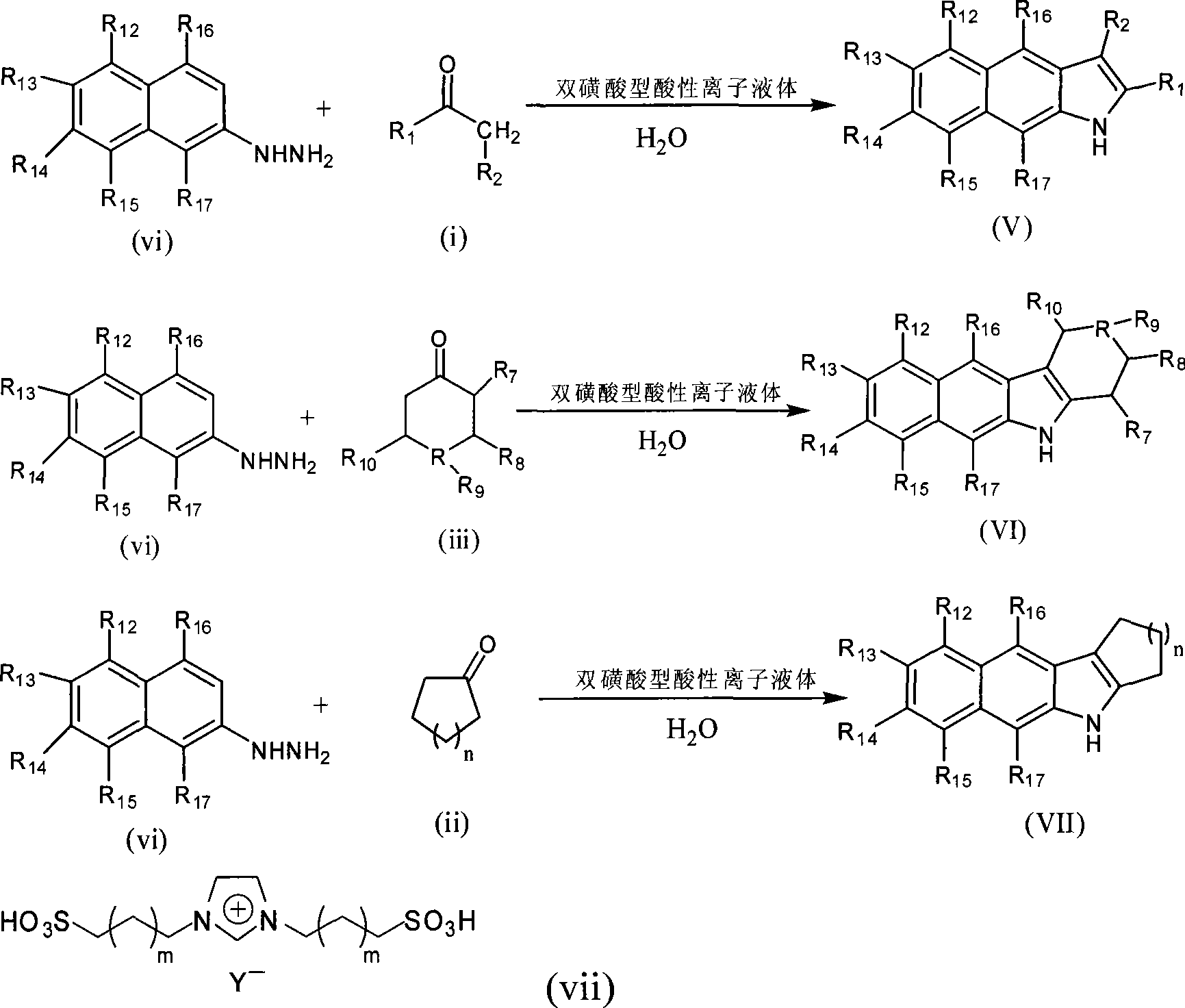
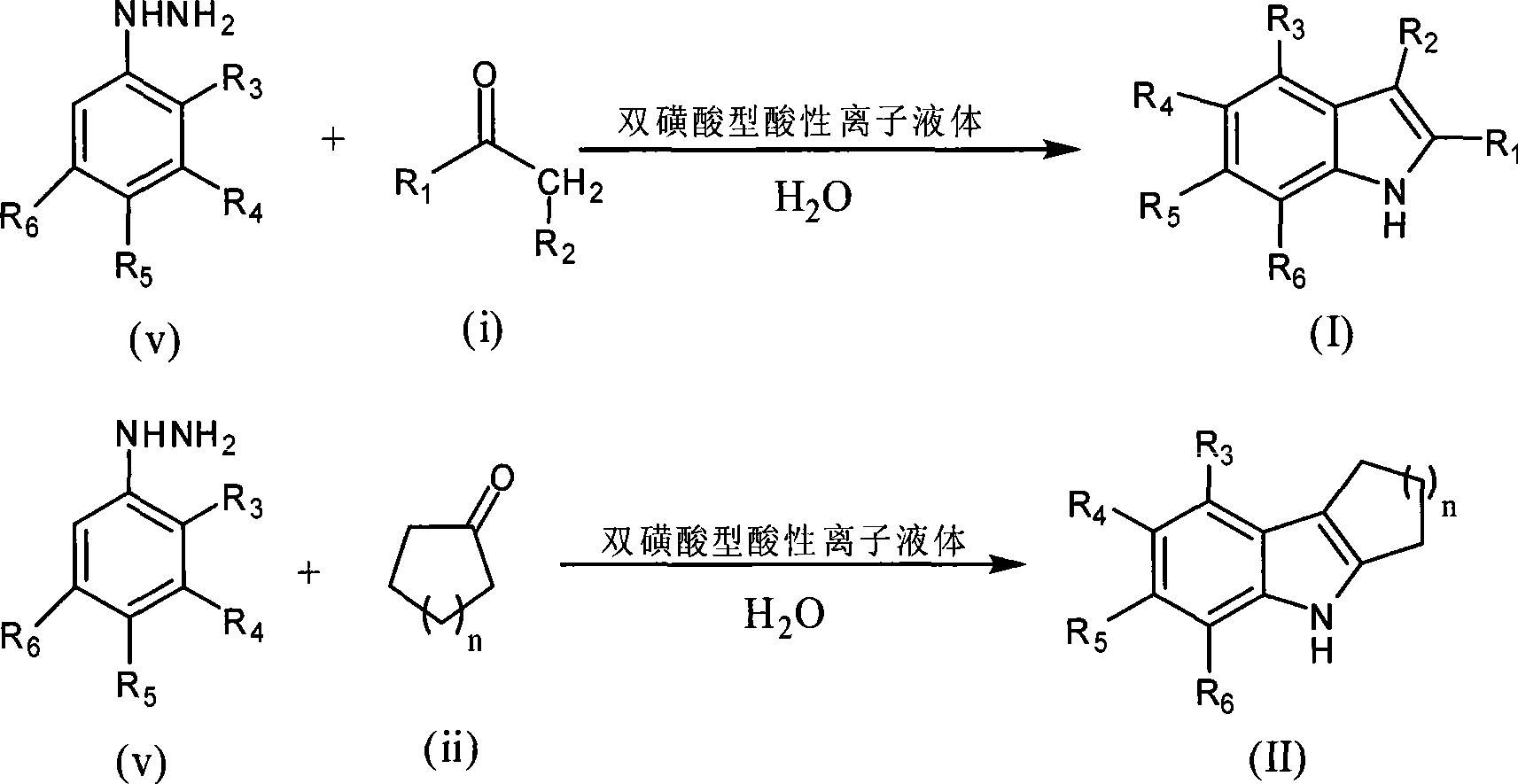
Green synthesis of indole compounds
A technology of green synthesis and synthesis method, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., can solve the problem of unfriendly solvents, strong corrosion of equipment, and catalyst dosage Large and other problems, to achieve the effect that the product yield is not affected, the product purity is good, and the cost is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Double sulfonic acid type acidic ionic liquid [(HSO 3 -p) 2 im][HSO 4 ](2.5mmol) was added to a 25ml double-necked round bottom flask containing water (15ml), and mechanically stirred into a homogeneous ionic liquid aqueous solution, cyclohexanone (0.490g, 5mmol) and phenylhydrazine (0.540g, 5mmol) in sequence Add to the reaction vessel, under mechanical stirring, heat in an oil bath at 70°C, keep the temperature for 0.5h, and cool to room temperature. The mixture is directly filtered and dried to obtain 0.83g of 1,2,3,4-tetrahydrocarbazole. The yield is 93%, and the product structure is:
[0033]
Embodiment 2
[0035] Double sulfonic acid type acidic ionic liquid [(HSO 3 -p) 2 im][HSO 4 ](2.5mmol) was added to a 25ml double-necked round bottom flask filled with water (30ml), mechanically stirred evenly into a homogeneous ionic liquid aqueous solution, cyclohexanone (0.490g, 5mmol) and phenylhydrazine (0.540g, 5mmol) in sequence Add to the reaction vessel, under mechanical stirring, heat in an oil bath at 70°C, keep the temperature for 1 hour, cool to room temperature, directly filter and dry the mixture to obtain 0.76 g of 1,2,3,4-tetrahydrocarbazole. The rate is 87%, and the product structure is:
[0036]
Embodiment 3
[0038] Double sulfonic acid type acid ionic liquid [(HSO 3 -p) 2 im][HSO 4 ](2.5mmol) was added to a 25ml double-necked round bottom flask containing water (15ml), and mechanically stirred into a homogeneous ionic liquid aqueous solution, cyclohexanone (0.490g, 5mmol) and phenylhydrazine (0.540g, 5mmol) in sequence Add to the reaction vessel and react at room temperature for 12 hours under mechanical stirring. The mixture is directly filtered and dried to obtain 0.83 g of 1,2,3,4-tetrahydrocarbazole with a yield of 90%. The product structure is:
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com