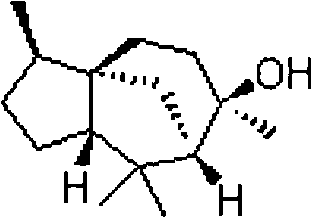
New medical application of cedrol
A kind of cedar alcohol, the technology of use, applied in the new use field in analgesia and treatment of arthritis medicine, achieve the effect of good analgesia and anti-inflammatory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
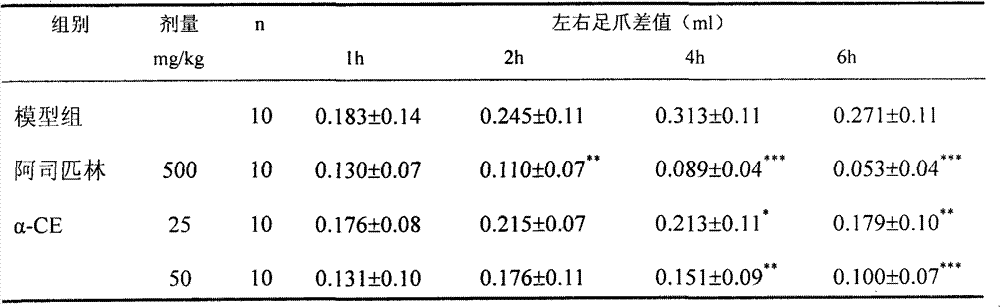
[0026] Example 1: Effects on carrageenan-induced paw edema and inflammation in rats
[0027] Method: 60 SD rats, body weight: ♀140~176g, 150~180g, ♀ half and half, divided into 6 groups, 10 in each group. The model group was intragastrically administered with 1% TW-80, and the test drug α-CE was divided into 4 dosage groups of 100mg / kg, 50mg / kg, 25mg / kg and 10mg / kg, administered intragastrically (ig) once a day , a total of 4 days of administration. Positive control drug is aspirin 0.5g / kg only before the test by intragastric administration (ig) once. After ig 1h on d4, sc 0.1ml of 0.5% carrageenan was given to the right hind paw of the rat. The change of paw volume was measured by capillary method at 1h, 2h, 4h, 6h after sc. The results were statistically processed by computer SigmaStat software, and the significant difference between aspirin and model group (1%TW-80) was compared.
[0028] Result: α-CE25mg / kg, 50mg / kg, 100mg / kg and aspirin 0.5g / kg have significant in...
Embodiment 2
[0032] Example 2: Effects on Adjuvant Arthritis in Rats
[0033] Methods: 60 SD rats were taken and divided into 5 groups on average according to body weight: (1) 1% TW-80 (vehicle); (2) positive control drug dexamethasone phosphate injection 2.5 mg / kg; (3) α - CE 25 mg / kg; (4) α-CE 50 mg / kg; (5) α-CE 100 mg / kg. All were administered by intragastric administration (ig). 0.1 ml of complete Freund's adjuvant (containing 0.5 mg of BCG) was injected into the right hind paw. Oral administration once 30 minutes before the injection of adjuvant. 18 hours after the injection of the adjuvant, measure the swelling of the right hind paw, observe the preventive effect of CE on early acute inflammation, and then stop the drug; one week later, start intragastric administration, once a day, for 7 consecutive days, and measure once on d15 Observe the preventive effect of CE on secondary lesions for the swelling of the left hind foot, and then stop the drug; start therapeutic administration...
Embodiment 3
[0047] Example 3: Effects on Tail Flick of Rats Caused by Photothermal Stimulation (Photothermal Analgesia Model)
[0048] Methods: 1) Dose selection and grouping: 1% TW-80 was used as negative control, and indomethacin 10 mg / kg was used as positive control. The mixture of α-CE and β-CE has three dosage groups of 12.5mg / kg, 25mg / kg and 50mg / kg. 10 rats per group, ♀ Half and half were kept in separate cages. 2) Administration: intragastric administration (ig) once, the volume of intragastric administration is 1ml / 100g. 3) Observation: 1 hour after the ig, the tail of the rat was stimulated by light and heat, and the time for the rat to flick the tail was recorded. The results were statistically processed by computer SigmaStat software, and the significant differences were compared between indomethacin, CE and 1% TW-80 groups.
[0049] Results: CE12.5mg / kg, 25mg / kg, 50mg / kg and indomethacin 10mg / kg had significant analgesic effects on the tail of rats stimulated by light an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com