Intraocular implant for preventing and treating after cataract and preparation method thereof
A technology of implants and preparations, applied in the field of medicine, which can solve the problems of low drug concentration, low drug efficacy, and short duration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The weight ratio of docetaxel to MMA (methyl methacrylate), HEMA (hydroxyethyl methacrylate) cross-linked copolymer is 1:50
[0038] The ratio of MMA to HEMA is 20:80
[0039] 15 μl of cross-linker EDGMA
[0040] Preparation steps:
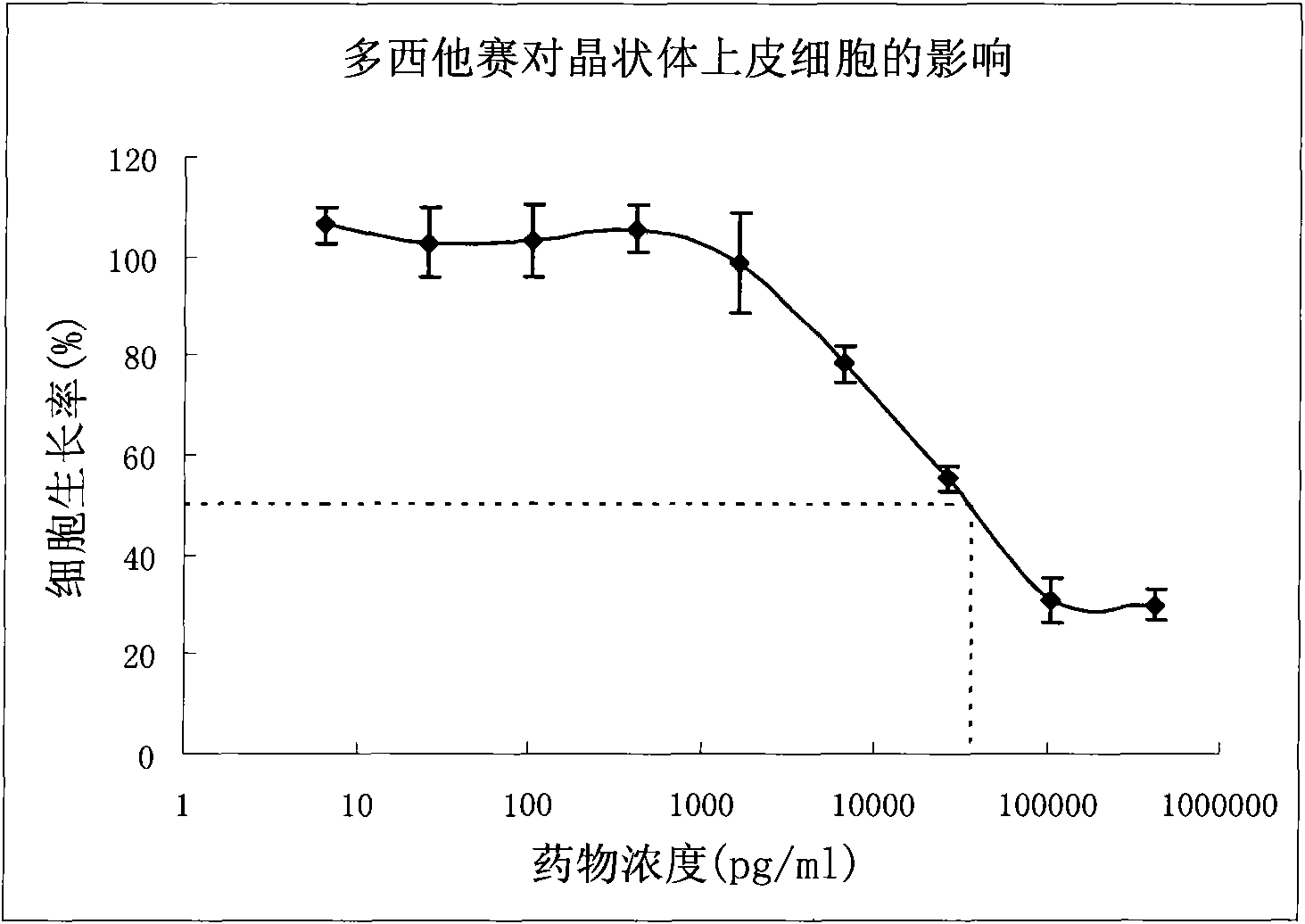
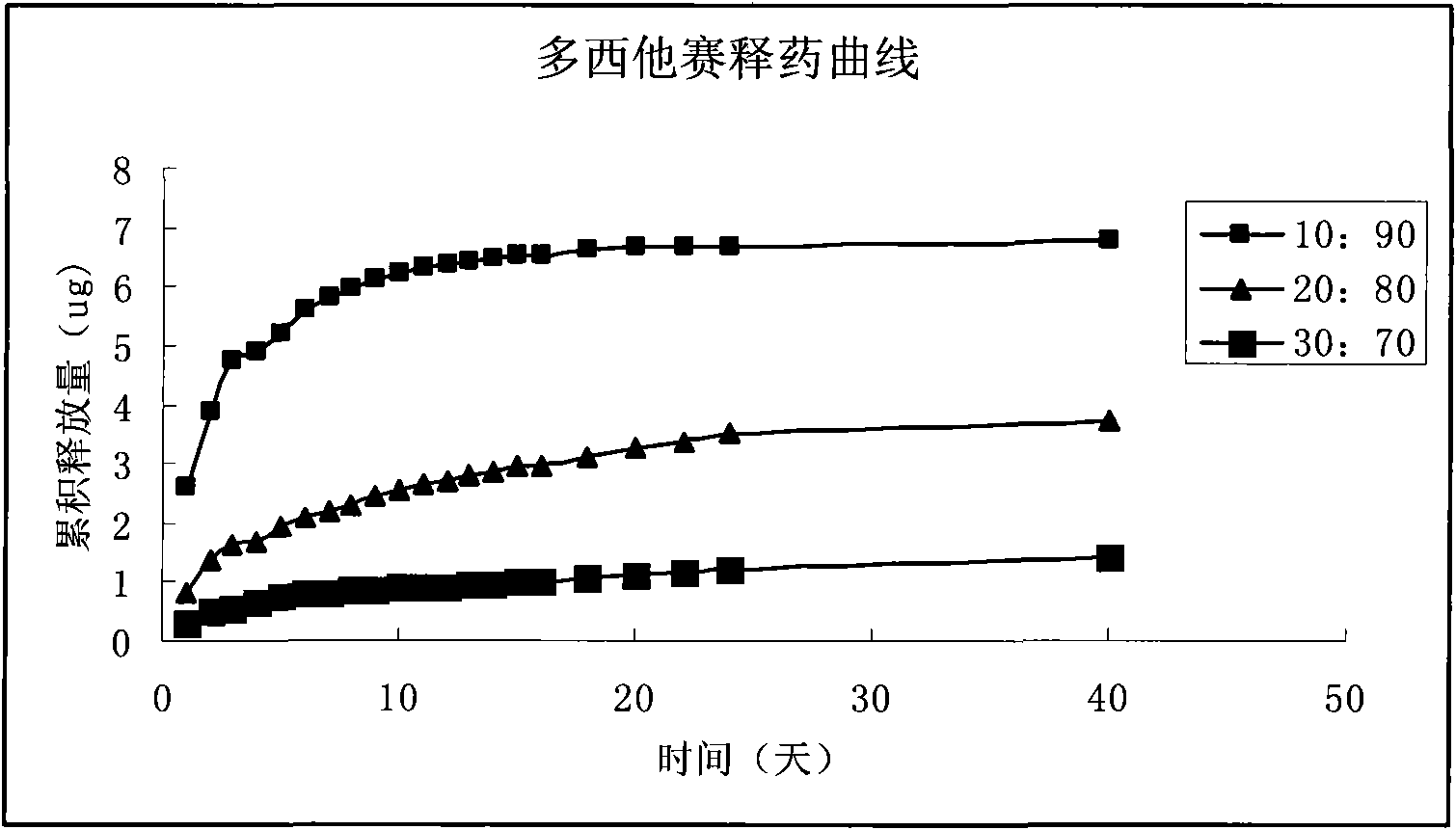
[0041] Weigh 100 mg of docetaxel, 1 ml of MMA and 4 ml of HEMA, add 15 μl of cross-linking agent EDGMA and 19 mg of initiator AIBN, sonicate until all solids are completely dissolved, pass through nitrogen protection, pour into a mold, and react at 60°C for 4 day, removed and sterilized. With the phosphate buffer of pH=7.4 as the medium, the in vitro drug release of the preparation was measured, and the results are shown in image 3 .
Embodiment 2
[0043] The specific gravity of docetaxel and auxiliary materials is 1:50, the ratio of slow-release polymer MMA to HEMA is 10:90, 15 μl of cross-linking agent and 19 mg of initiator are added, and the preparation steps are as in Example 1 to prepare docetaxel eye Sustained-release implants. With the phosphate buffer of pH=7.4 as the medium, the in vitro drug release of the preparation was measured, and the results are shown in image 3 .
Embodiment 3
[0045] The specific gravity of docetaxel and auxiliary materials is 1:50, the ratio of sustained-release polymer MMA to HEMA is 30:70, 15 μl of cross-linking agent and 19 mg of initiator are added, the preparation steps are as in Example 1, and the docetaxel eye is prepared. Sustained-release implants. With the phosphate buffer of pH=7.4 as the medium, the in vitro drug release of the preparation was measured, and the results are shown in image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com