Drug formulation containing fibrate medicament and process for producing the same
A technology for fibrates and medicines, which is applied in the field of preparations containing fibrates and their preparation, and can solve the problem that the mixing ratio of pitavastatin and fenofibrate is not recorded, the relationship between free fatty acids is not mentioned, and the effect is not fully demonstrated. and other problems, to achieve the effect of reducing the concentration of fibrinogen and inhibiting thrombosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1 (experimental example)
[0079] Pitavastatin (PIT) was mixed into the feed, and administered to normolipidemic rabbits at a dose of 0.5 mg / kg / day for 4 weeks (PIT administration group). Fenofibrate (FEN) was then mixed into the feed, and administered to normal blood lipid rabbits at a dose of 30 mg / kg / day for 4 weeks (FEN administration group). Then PIT and FEN were mixed into feed and administered to normal blood lipid rabbits for 4 weeks (combined administration group). In the combination administration group, the dosage of PIT was 0.5 mg / kg / day, and the dosage of FEN was 30 mg / kg / day.
[0080] After the last administration, the rabbits were fasted for one day, blood was collected from the ear vein, and treated with ethylenediaminetetraacetic acid (EDTA) to prepare plasma. Total cholesterol (TC) and triglyceride (TG) concentrations in plasma were measured according to an enzymatic method using a detection kit for enzymatic method (manufactured by Wako P...
Embodiment 2
[0082] Embodiment 2 (experimental example)
[0083]Pitavastatin (PIT) was orally administered to normal guinea pigs at a dose of 1 mg / kg / day for 2 weeks (PIT administration group). Fenofibrate (FEN) was orally administered to normal guinea pigs at a dose of 30 mg / kg / day for 2 weeks (FEN administration group). PIT and FEN were then orally administered to normal guinea pigs for 2 weeks (combined administration group). In the combination administration group, the dosage of PIT was 1 mg / kg / day, and the dosage of FEN was 30 mg / kg / day.
[0084] After the last administration, the guinea pigs were fasted for 1 day, and the blood collected from the abdominal aorta was made into plasma as in Example 1, and the TC and TG values were measured, and the cholesterol content in the liver was measured.
[0085] The results showed that TC and TG were significantly decreased in the combined administration group in which both PIT and FEN were administered, compared to the PIT-administered gro...
Embodiment 3
[0086] Embodiment 3 (experimental example)
[0087] Study on the effects of reducing blood lipids in dogs caused by combined administration of fenofibrate (FEN) and pitavastatin (PIT)
[0088] (1) Subject
[0089] Experiments were carried out with dogs (male and male Beagle dogs used for drug research, 11 males and 2 females, aged 27 to 73 months). Dogs were raised in separate cages in an animal room set at room temperature 23±3°C, humidity 50±10%, light period 8:00-20:00 and dark period 20:00-8:00, one per cage. Feed the dog with 300g CD-5M feed (manufactured by Japan CLEA) once a day. Make it ad libitum to drink tap water.
[0090] (2) Tested object and control object
[0091] Fenofibrate (FEN) 20 mg / kg and pitavastatin (PIT) 2 mg / kg were used as test substances.
[0092] (3) experiment
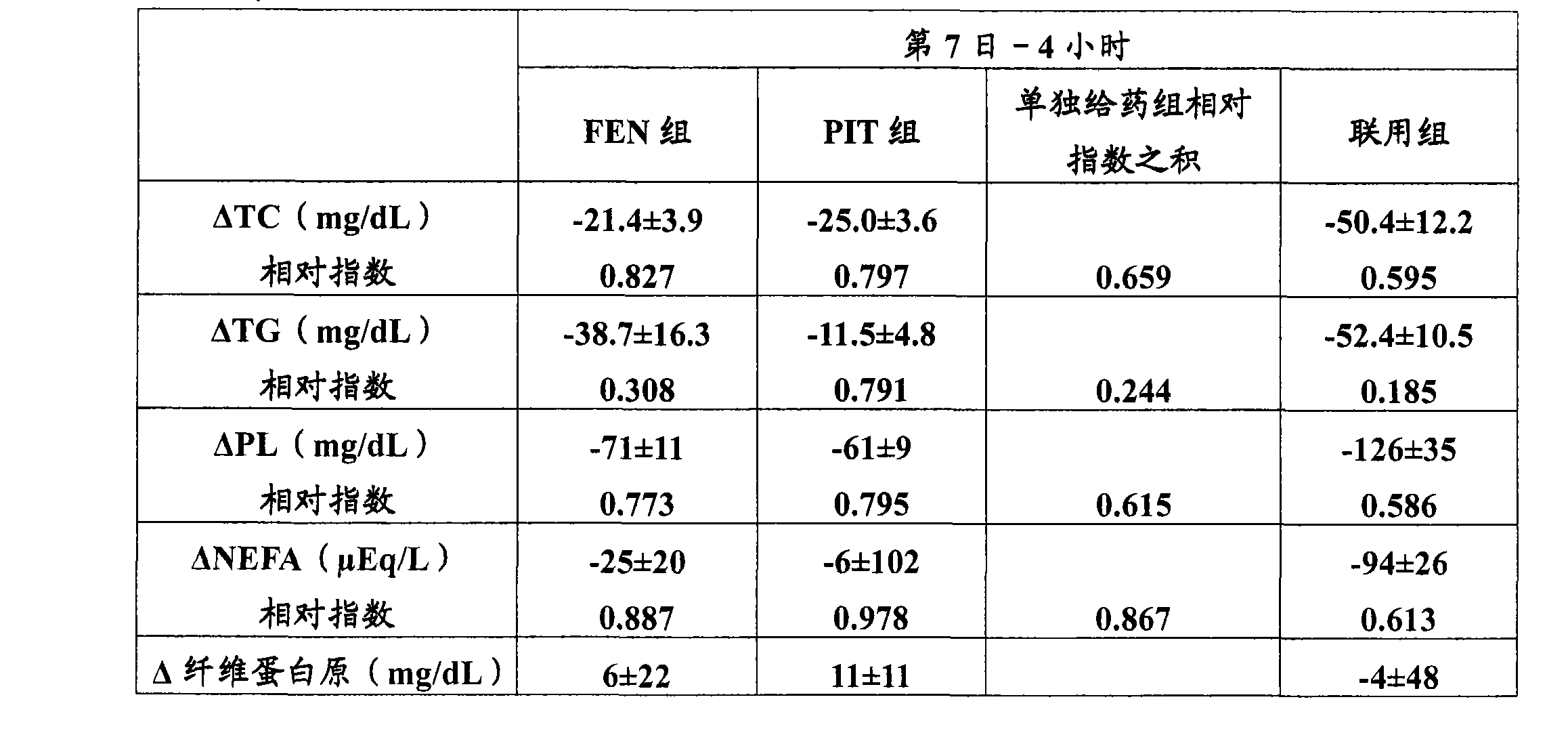
[0093] One day before the start of administration, blood was collected from the anterior wrist radial vein at 4 hours (the 0th day-4 hours) and 24 hours (the 0th day-24 hours) after f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com