Conversion of glycerine to dichlorohydrins and epichlorohydrin
A technology of dichloropropanol and epichlorohydrin, which is applied in the field of conversion of glycerin to dichloropropanol and epichlorohydrin, and can solve problems affecting economy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
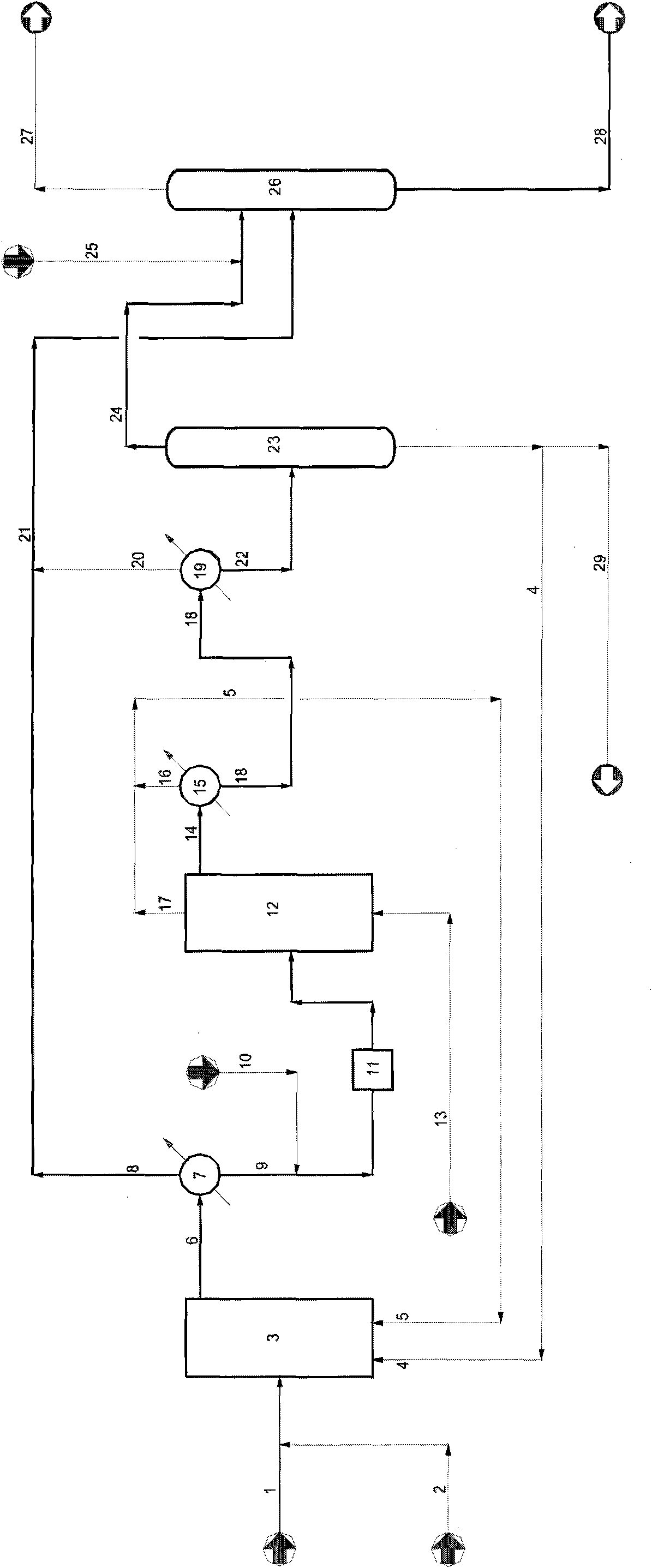
Image
Examples
Embodiment 1
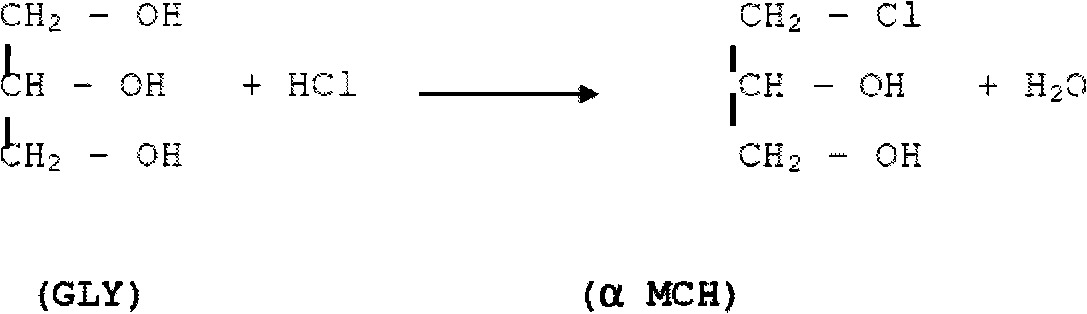
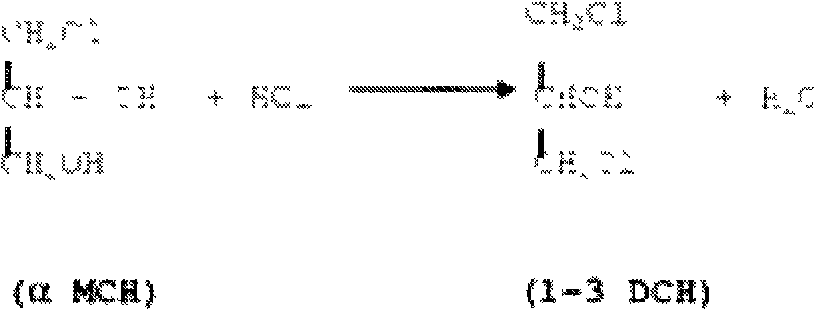
[0077] Embodiment 1 utilizes acetic acid as catalyst to prepare DCH by GLY
[0078] The reactor had been charged with 150 g of GLY containing 6 mole % acetic acid.
[0079] Gaseous HCl was charged as needed to maintain a constant pressure of 2 bar at 100°C.
[0080] After one hour, at a temperature of 100° C., the pressure in the reactor was increased to 9 bar.
[0081] The reaction was allowed to proceed for another 3 hours. Periodically samples were taken and analyzed.
[0082] GC analysis performed after each reaction stage to remove water, catalyst and residual hydrogen chloride showed the following distribution:
[0083] 1 hour later 4 hours later
[0084] GLY 11.48 NEGL.
[0085] α-MCH 77.55 0.76
[0086] β-MHC 4.94 8.08
[0087] 1.3 DCH 6.03 89.62
[0088] 1.2DHC NEGL. 1.54
Embodiment 2
[0089] Embodiment 2 utilizes malic acid as catalyst to prepare DCH by GLY
[0090] The reaction was carried out in the same manner as in Example 1, except that 8 mol % of malic acid was used as a catalyst with respect to GLY. GC analysis performed after each reaction stage showed the following distribution:
[0091] 1 hour later 4 hours later
[0092] GLY 7.63 NEGL.
[0093] α-MCH 83.55 27.43
[0094] β-MHC 5.65 8.20
[0095] 1.3 DCH 3.17 63.23
[0096] 1.2DHC NEGL. 1.14
Embodiment 3
[0097] Embodiment 3 utilizes malic acid and acetic acid as catalyst to prepare DCH by GLY
[0098] The reaction was performed analogously to Example 2, except that after 1 hour and just before pressurization after flashing at near atmospheric pressure, an additional 3 mole % acetic acid relative to GLY was added to the reaction mass.
[0099] The reaction was complete in a shorter additional time (2 hours and 20 minutes instead of 3 hours in Examples 1 and 2).
[0100] GC analysis performed after each reaction stage showed the following distribution:
[0101] 1 hour later 3 hours 20 minutes later
[0102] GLY 18.23 NEGL.
[0103] α-MCH 76.40 NEGL.
[0104] β-MHC 5.37 4.82
[0105] 1.3DCH NEGL. 92.99
[0106] 1.2DHC NEGL. 2.19
[0107] Comparing the results, the following points are obtained:
[0108] 1) Acetic acid is the most active single catalyst, allowing a high degree of conversion within a given time. However, due to the relatively high volatility of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com