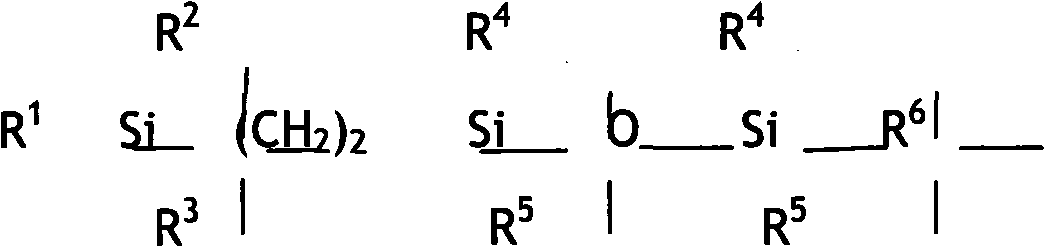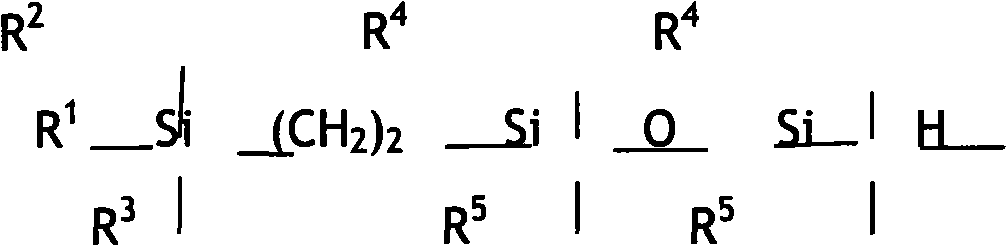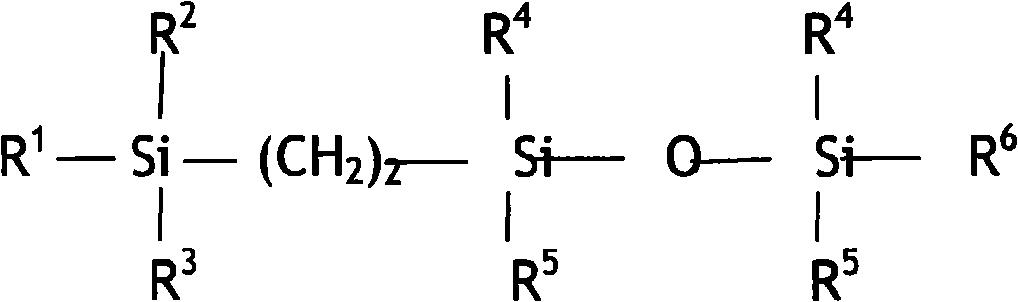Disiloxane compound terminated by trialkyl silica ethyl and alkyne enol and method for preparing same
A technology of alkylsilylethyl and disiloxane, which is applied in the direction of silicon organic compounds, botanical equipment and methods, non-ionic surface active compounds, etc., and can solve problems such as the instability of trisiloxane compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of Trimethylsilylethyltetramethylhydrogendisiloxane (Intermediate 1):
[0030] Add tetramethyldisiloxane 103.2g in a 500 milliliter bottom four-neck round bottom flask with electric heating, Wilkinson catalyst ((PPh 3 ) 3 RhCl) 200ppm, under reflux and condensing, stir with nitrogen gas and heat up to 60°C, add 51.2g of trimethylvinylsilane dropwise at a rate of 1g / min at a reaction temperature of ≤70°C through a dropping funnel, and maintain at 65°C after the addition is complete After the reaction was completed for 1 hour, the product was distilled under reduced pressure (30 mmHg) under vacuum conditions to obtain 100 g of the target product.
[0031] Molecular structure of intermediate 1:
[0032]
Embodiment 2
[0034] Preparation of tert-butyldimethylsilylethyltetramethylhydrogendisiloxane (Intermediate 2):
[0035] Add tetramethyldisiloxane 134g, Wilkinson catalyst ((PPh 3 ) 3 RhCl) 200ppm, under reflux and condensing, stir with nitrogen gas and heat up to 60°C, add 142g of tert-butyldimethylvinylsilane dropwise at a reaction temperature of ≤70°C through the dropping funnel at a rate of 1g / min, after the addition is completed, the reaction temperature is 65 The reaction was maintained at ℃ for 2 hours. After the reaction was completed, the product was distilled under reduced pressure (30 mmHg) under vacuum conditions to obtain 140 g of the target product.
[0036] Molecular structure of intermediate 2:
[0037]
Embodiment 3
[0039] Preparation of isopropyldimethylsilylethyltetraethylhydrogendisiloxane (Intermediate 3):
[0040] Add tetraethylhydrogendisiloxane 190g in a 500 milliliter bottom four-neck round bottom flask with electric heating, Wilkinson catalyst ((PPh 3 ) 3 RhCl) 200ppm, under reflux and condensing, stir with nitrogen gas and heat up to 70°C, add 128g of isopropyldimethylvinylsilane dropwise at a reaction temperature of ≤80°C through a dropping funnel at a rate of 1g / min. The reaction was maintained at ℃ for 1 hour. After the reaction, the product was distilled under reduced pressure (30 mmHg) under vacuum conditions to obtain 150 g of the target product.
[0041] Molecular structure of intermediate 3:
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com