Method for purifying rna on a preparative scale by means of hplc
A technology of mobile phase and organic solvent, which is applied in the application field of porous reversed phase as a stationary phase in this method, can solve complex and expensive gradient procedures and other problems, and achieve the effects of simple recovery, sensitive detection, and easy automatic operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
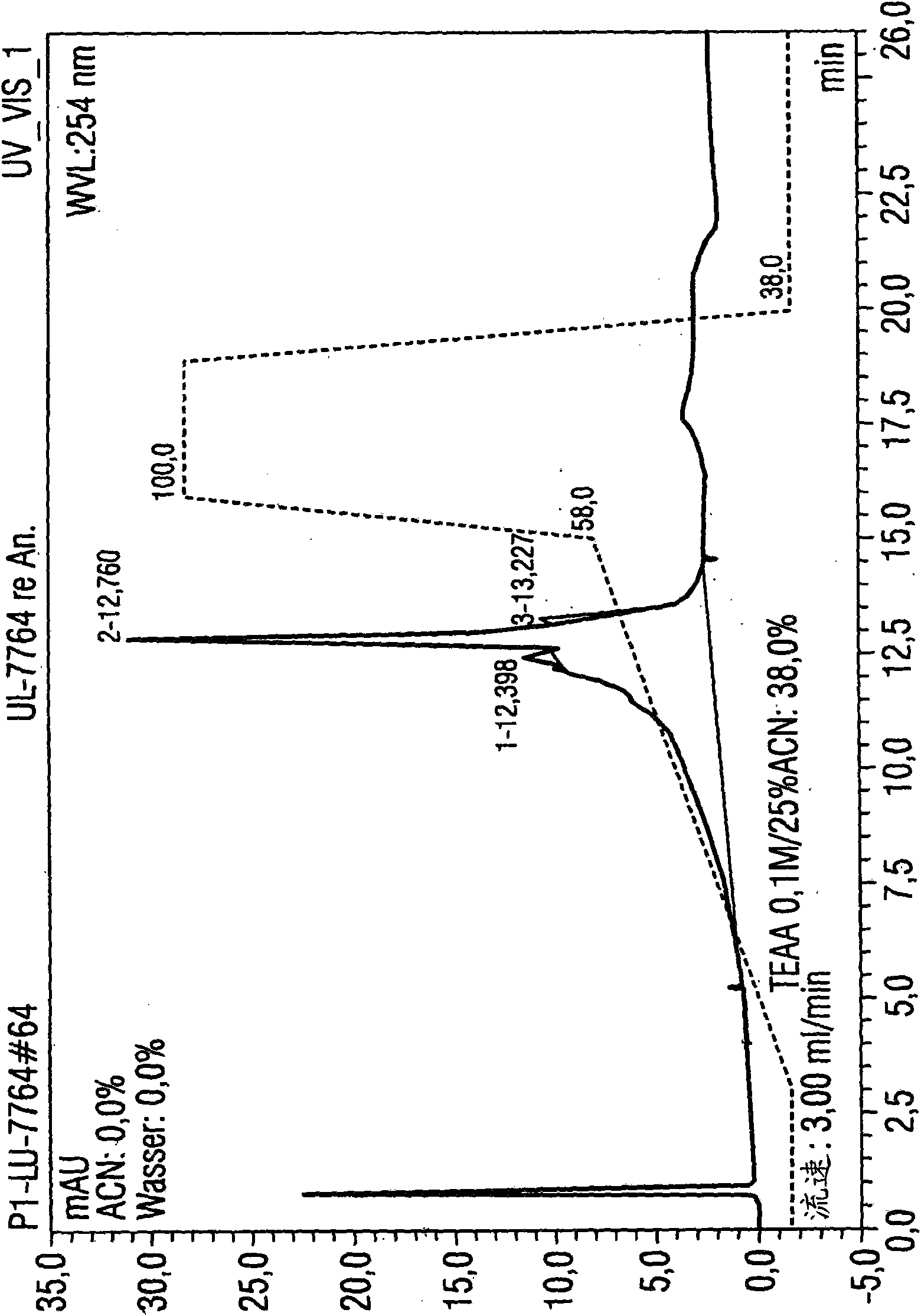
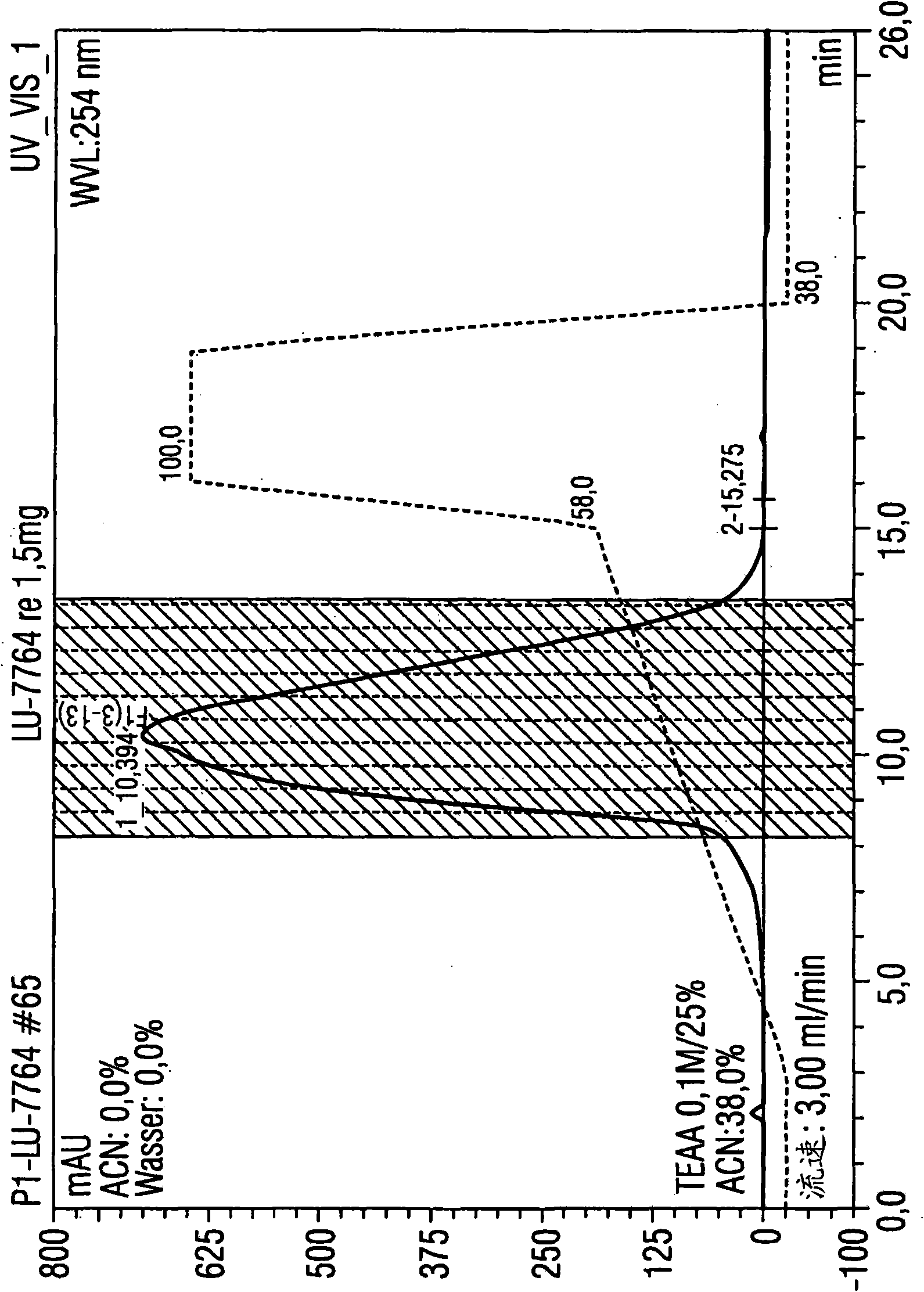
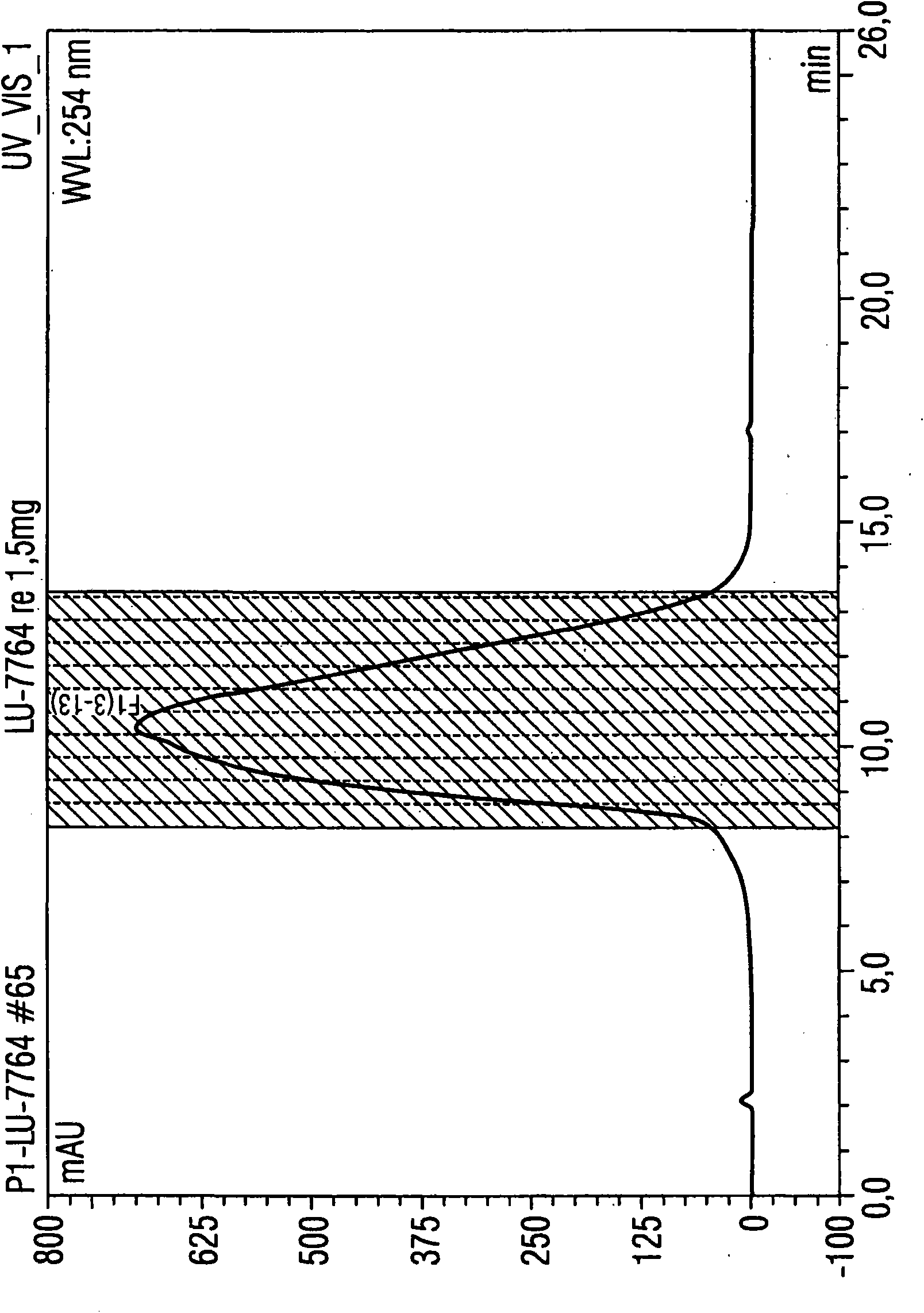
[0089] Example 1: Utilize the HPLC method according to the present invention to purify 1.5 mg luciferase mRNA
[0090] Luciferase mRNA with a size of 1825 base pairs was used for isolation. A porous non-alkylated polystyrene / divinylbenzene (polystyrene divinylbenzene) matrix (commercially available from Polymer Laboratories) was used as the stationary phase. It has a particle size of 8 μm and a pore size of 4000 The columns used were 5.0 cm in length and 7.5 mm in diameter. The temperature of the injector was 12°C, and the temperature of the HPLC separation, specifically, the temperature of the separation column was 78°C, that is, the operation was performed under completely denaturing conditions.
[0091] Separation was performed by the following gradient program:
[0092] Eluent A: 0.1M triethylammonium acetate, pH7
[0093] Eluent B: 0.1M triethylammonium acetate, pH7, containing 25vol.% acetonitrile
[0094] Eluent composition:
[0095] Start: 62% A and 38% B (1st...
Embodiment 2
[0105] Example 2: With an aperture of 1000 The stationary phase separates 200 μg of 2kb and 4kb RNA fragments
[0106] 200 μg of 2kb and 4kb RNA fragments were used for isolation. A porous non-alkylated polystyrene / divinylphenyl substrate (commercially available from Polymer Laboratories) was used as stationary phase. It has a particle size of 8 μm and a pore size of 1000 The columns used were 2.5 cm long and 4.6 mm in diameter. The injector temperature was 12°C, and the HPLC separation temperature, specifically, the temperature of the separation column was 78°C, that is, the operation was performed under completely denaturing conditions.
[0107] Separation was performed using the following gradient program:
[0108] Eluent A: 0.1M triethylammonium acetate
[0109] Eluent B: 0.1M triethylammonium acetate / 25% acetonitrile
[0110] Eluent composition:
[0111] · Starting level: 62% A and 38% B (1st to 3rd minutes)
[0112] Separation range I: Gradient 38%-49.5% B (B...
Embodiment 3
[0117] Example 3: With an aperture of 4000 The stationary phase separates 100 μg of 2kb and 4kb RNA fragments
[0118] 100 μg of 2kb and 4kb RNA fragments were used for isolation. A porous non-alkylated polystyrene / divinylphenyl substrate (commonly available from Polymer Laboratories) was used as stationary phase. It has a particle size of 8 μm and a pore size of 4000 The columns used were 2.5 cm in length and 4.6 mm in diameter. The injector temperature was 12°C, and the HPLC separation temperature, specifically, the temperature of the separation column was 78°C, that is, the operation was performed under completely denaturing conditions.
[0119] Separation was performed using the following gradient program
[0120] Eluent A: 0.1M triethylammonium acetate
[0121] Eluent B: 0.1M triethylammonium acetate / 25% acetonitrile
[0122] Eluent composition:
[0123] · Starting level: 62% A and 38% B (1st to 3rd minute)
[0124] Separation range I: Gradient 38%-49.5% B (B ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com