Doxofylline-contained liquid injection, preparation method and quality control method thereof
A technology of doxofylline and water injection, applied in the field of water injection containing doxofylline, achieving the effect of flexible specification design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Specification: 10ml:0.2g
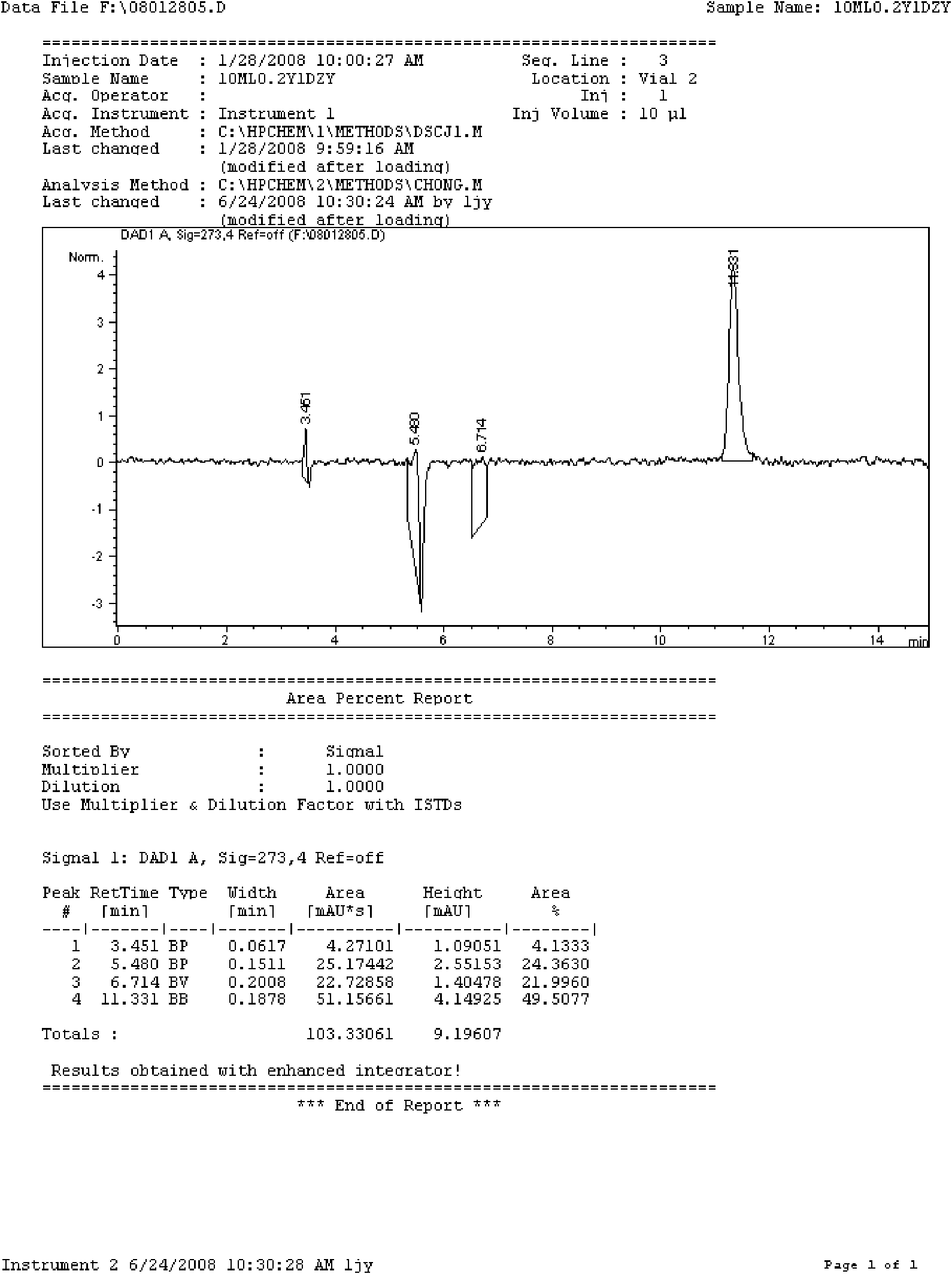
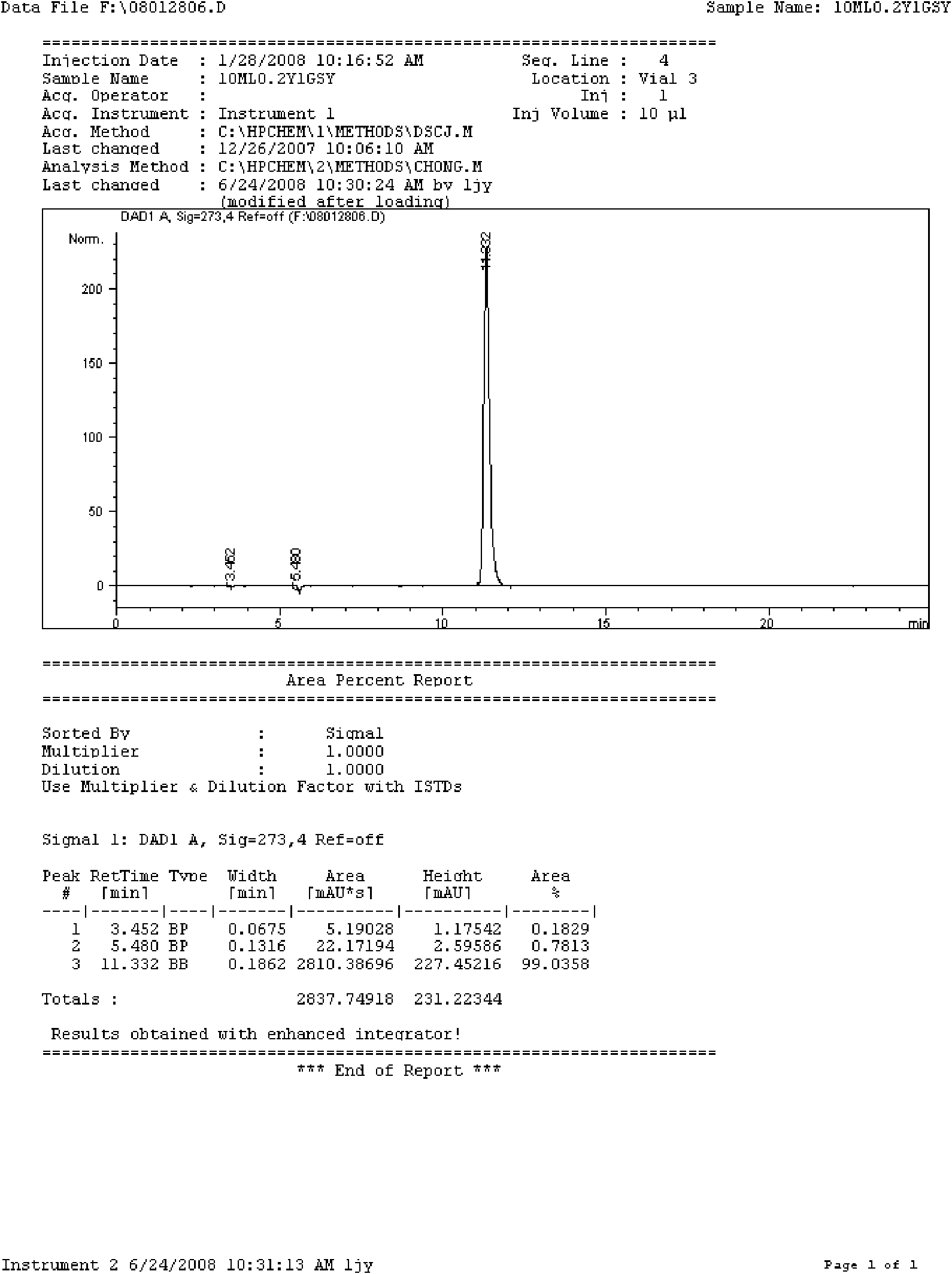
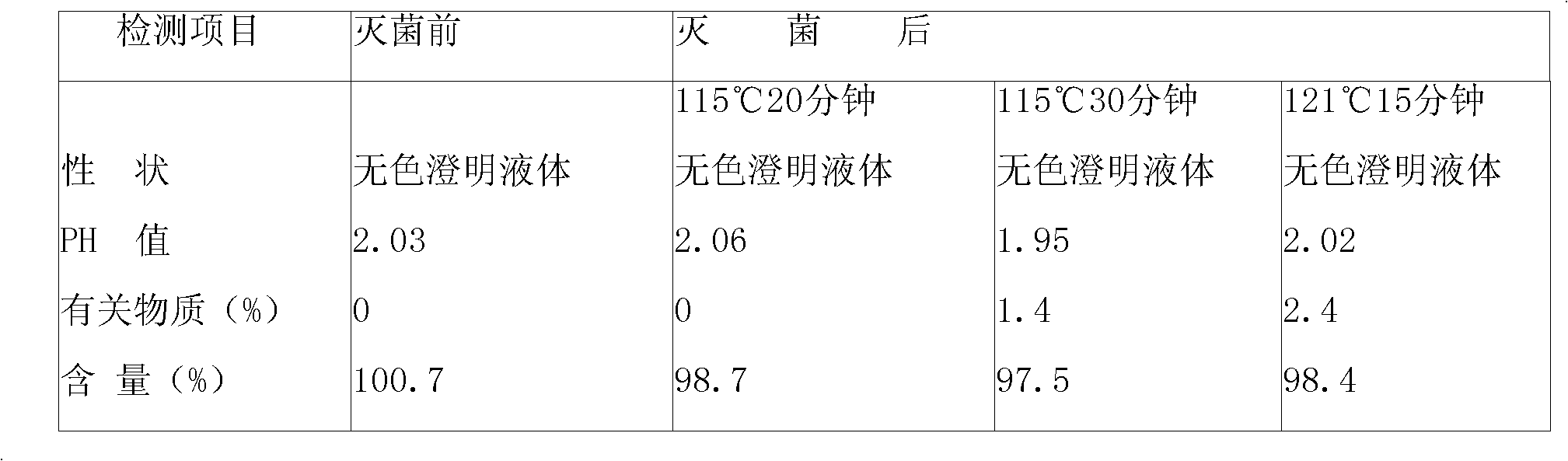
[0090] Take 4000ml of water for injection, heat up, add 200g of doxofylline under stirring, wait until the temperature of the liquid rises to about 80°C, add 20g of activated carbon after the powder is completely dissolved, continue stirring for 10 minutes, wait until the temperature drops to 40°C, filter to remove carbon , have a thick dosing solution. Take the concentrated solution and add water for injection to make up to 10000ml, stir evenly, filter through a microporous membrane until the clarity is qualified, then take a sample to check the content and pH value. The content should be 95-105% of the labeled amount, and the pH value should be 5.0-6.5. Put it in a 10ml ampoule in a clean area, seal it, place it in an autoclave, steam sterilize it at 115°C for 30 minutes, and get it after finished product inspection, printing, and packaging.
Embodiment 2
[0092] Specifications: 20ml:0.3g
[0093] Take 6000ml of water for injection, heat up, add 300g of doxofylline under stirring, wait until the temperature of the liquid rises to about 80°C, add 30g of activated carbon after the medicine powder is completely dissolved, continue stirring for 10 minutes, wait until the temperature drops to 40°C, filter to remove carbon , have a thick dosing solution. Take the concentrated solution and add water for injection to make up to 20000ml, stir evenly, filter through a microporous membrane until the clarity is qualified, then take a sample to check the content and pH value. The content should be 95-105% of the labeled amount, and the pH value should be 5.0-6.5. Put it in a 10ml ampoule in a clean area, seal it, place it in an autoclave, steam sterilize it at 115°C for 30 minutes, and get it after finished product inspection, printing, and packaging.
Embodiment 3
[0095] Specifications: 20ml:0.2g
[0096] Take 6000ml of water for injection, heat up, add 200g of doxofylline under stirring, wait until the temperature of the liquid rises to about 80°C, add 30g of activated carbon after the powder is completely dissolved, continue stirring for 10 minutes, wait until the temperature drops to 40°C, filter to remove carbon , have a thick dosing solution. Take the concentrated solution and add water for injection to make up to 20000ml, stir evenly, filter through a microporous membrane until the clarity is qualified, then take a sample to check the content and pH value. The content should be 95-105% of the labeled amount, and the pH value should be 5.0-6.5. Put it in a 10ml ampoule in a clean area, seal it, place it in an autoclave, steam sterilize it at 115°C for 30 minutes, and get it after finished product inspection, printing, and packaging.
[0097] Trial-produce pilot products according to the above process, the theoretical quantity of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com