Attenuation of vasoactive oxygen carrier-induced vasoconstriction
An oxygen-carrying carrier and vasoconstriction technology, applied in the fields of inorganic active ingredients, cardiovascular system diseases, blood diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] For a detailed review of the preparation and use of heme-based oxygen carriers see, for example, Spahn et al., (2005) Curr Pharm Des. 11(31):4099, Greenburg et al., (2004) Crit Care.8 Suppl 2:S61, and Artificial Oxygen Carrier: Its Front Line (Koichi Kobayashi et al., eds., 2005).
[0080] Nitric oxide gas administration
[0081] Administration of a vasoactive oxygen carrier (eg, a heme-based oxygen carrier such as a hemoglobin-based oxygen carrier) can induce systemic and pulmonary vasoconstriction in the recipient. As described in detail herein, inhalation of nitric oxide gas before and optionally after administration of the vasoactive oxygen carrier can prevent or reduce the occurrence of vasoconstriction that would otherwise occur after administration of the artificial oxygen carrier.
[0082] Safe and effective methods of administering nitric oxide are described, for example, in Zapol, US Patent 5,570,683; Zapol et al, US Patent 5,904,938; Bach et al, US Patent Ap...
Embodiment 1
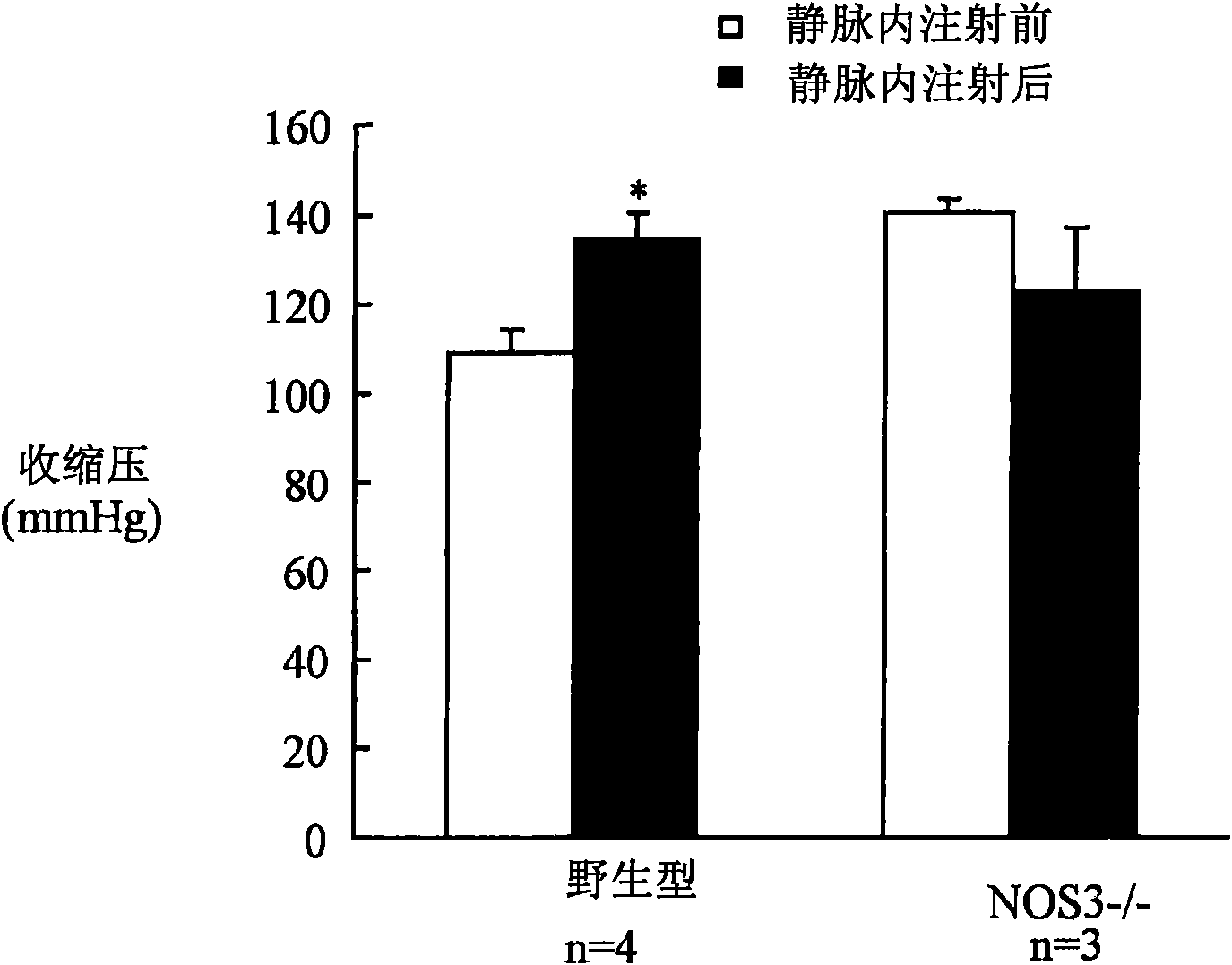
[0111] Example 1: Nitric oxide-dependent increase in blood pressure following infusion of a hemoglobin tetramer solution
[0112] Nitric oxide synthase 3 (NOS3) is expressed in pulmonary vascular endothelial cells and participates in the control of pulmonary vascular tone by synthesizing nitric oxide, which stimulates vascular smooth muscle cGMP synthesis and causes vasodilation. NOS3-deficient mice (NOS3- / -) exhibit systemic and pulmonary hypertension under normoxic conditions (Huang et al., (1995) Nature 377:239; and Steudel et al., (1997) Circ. Res. 81:34).
[0113] animal preparation
[0114] 8- to 10-week-old male C57BL / 6 wild-type mice and male NOS3-deficient mice (B6129P2-NOS3 tml / Unc ;NOS3 - / - )research. All mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and maintained in the Animal Resources Facility of the Massachusetts General Hospital.
[0115] Preparation of Mouse Hemoglobin Tetramer Solution
[0116] Murine whole blood was collected from wi...
Embodiment 2
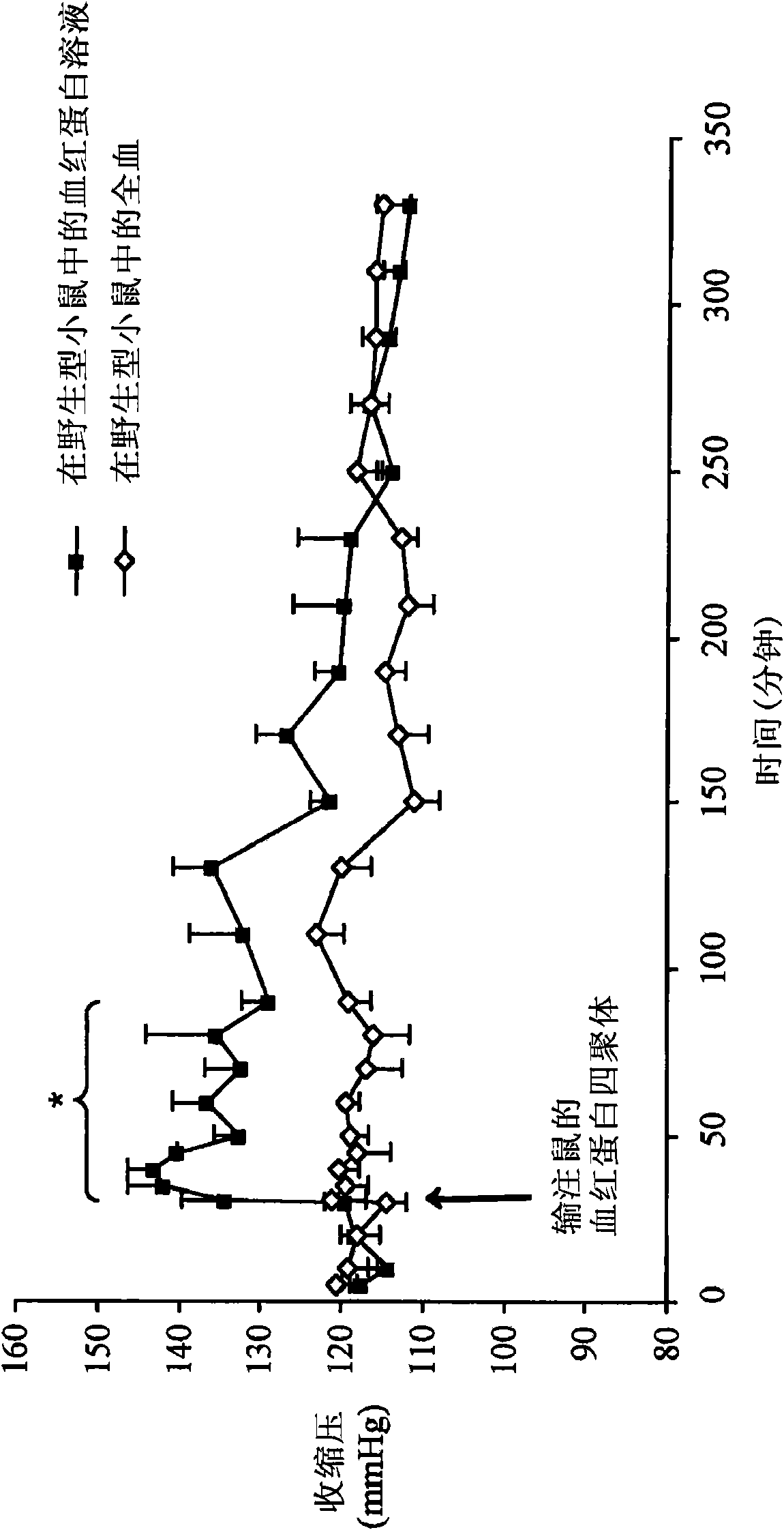
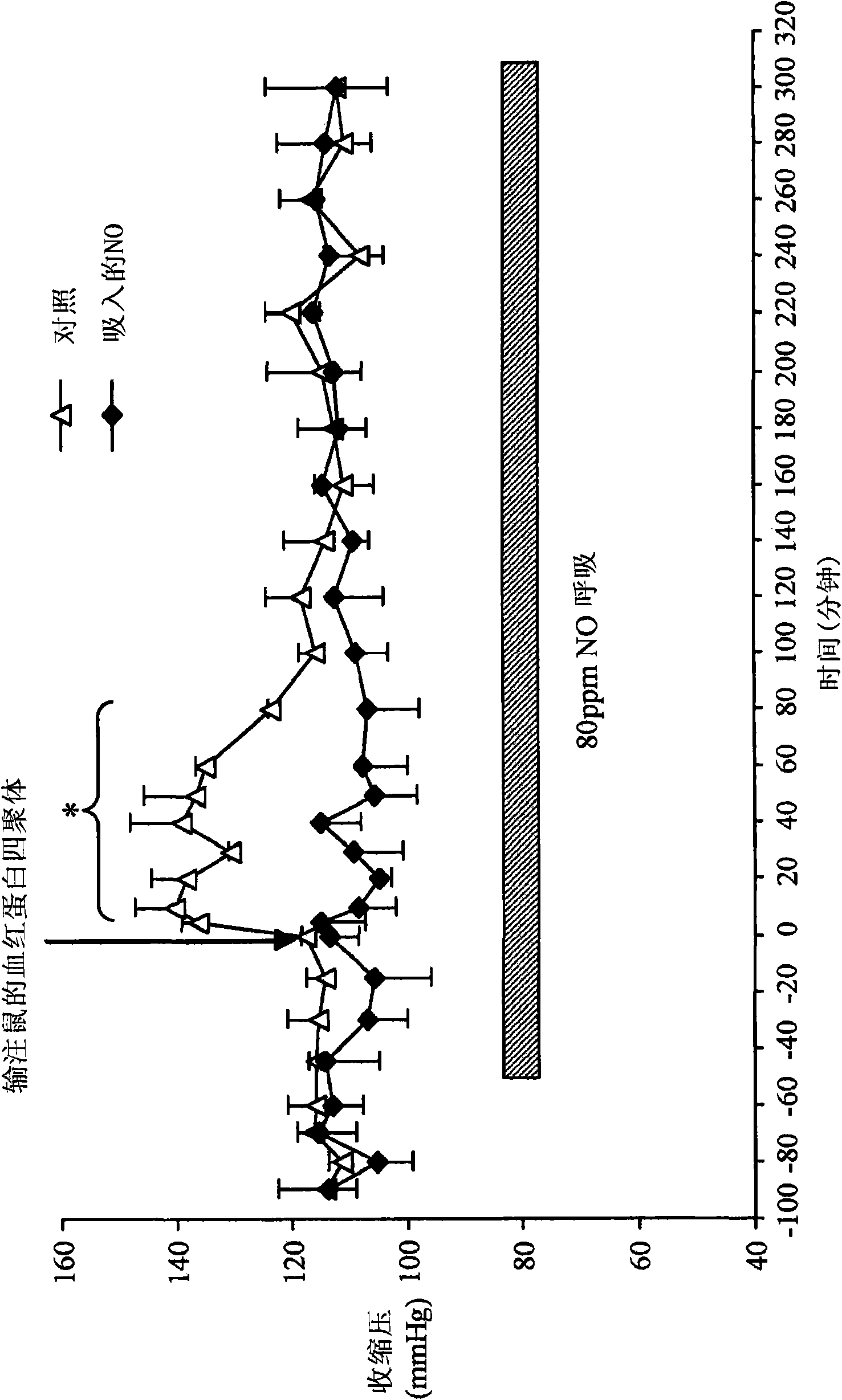
[0133] Example 2: Continuous Nitric Oxide Inhalation Prevents Increase in Systolic Blood Pressure and Converts Plasma Hemoglobin to Methemoglobin Before and After Infusion of Hemoglobin Tetramer Solution
[0134] Tail systolic pressure was measured in conscious wild-type mice (n=4) after intravenous injection of 0.012 ml / gram body weight of a solution of murine tetrameric hemoglobin. The "NO inhalation" group inhaled 80 ppm nitric oxide in air and the "control" group inhaled air. Continuous breathing of 80 ppm nitric oxide before and after infusion of mouse hemoglobin tetramer solution prevented hemoglobin-induced systolic blood pressure increase ( image 3 ).
[0135] Intravenous injection of 0.012 mL / g body weight while breathing air (n=4), breathing 80 ppm nitric oxide in air (n=4), or breathing 8 ppm nitric oxide in air (n=7) Methemoglobin concentrations in plasma were measured after mouse hemoglobin tetramer solution was administered to wild-type mice. Administration o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com