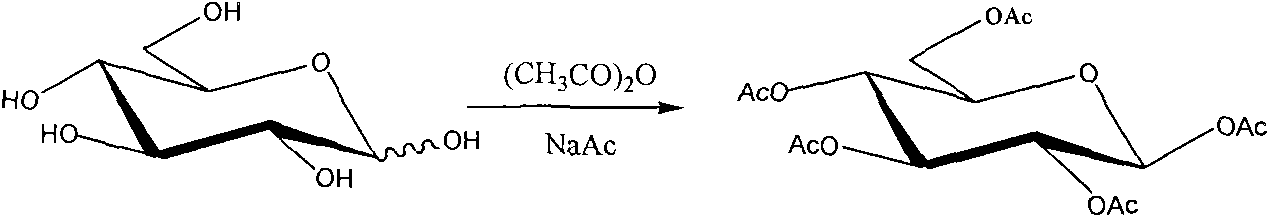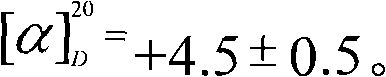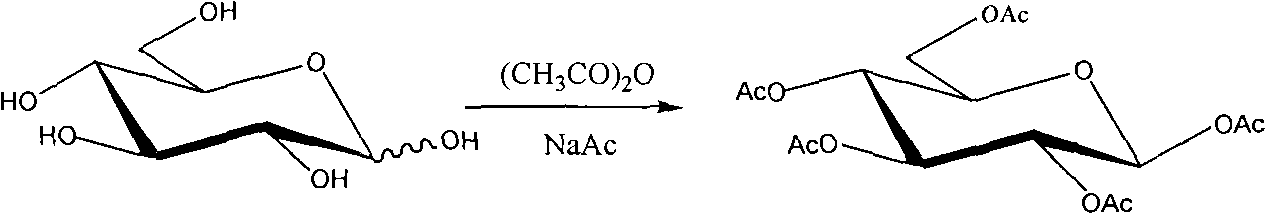Synthetic method of beta-1,2,3,4,6-penta-acetyl-D-glucopyranose
A technology of glucopyranose and pentaacetyl, applied in the field of β-1, can solve the problem of low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Weigh 4.00 g of anhydrous sodium acetate and add it to a 100 mL round-bottomed three-neck flask, then add 25.00 mL of acetic anhydride, and stir. Heat to reflux with an oil bath, and after the solution becomes clear in 30 minutes, add a total of 5.00 g of anhydrous D-glucose within 30 minutes, then control the temperature of the oil bath at 130°C and continue heating for 2 hours to obtain a brown-yellow solution. Pour the reaction solution into a beaker filled with 250mL of ice water. A large amount of crystals precipitated in the lower part of the beaker. After stirring for 2 hours, let it stand, pour off the upper solution, wash the crystals twice with ice water, filter, collect the crystals, and dry to obtain a white crude product. 10.83 grams, the product was dissolved in absolute ethanol and refluxed for 0.5h, cooled, placed in ice water to precipitate crystals, filtered and dried. 8.10 g of the target product were obtained with a yield of 74.72% and a melting poin...
Embodiment 2
[0027] Weigh 4.00 g of anhydrous sodium acetate and add it to a 100 mL round-bottomed three-neck flask, then add 25.00 mL of acetic anhydride, and stir. Heat to reflux with an oil bath, and after the solution becomes clear for about 30 minutes, add a total of 5.00 g of anhydrous D-glucose within 30 minutes, then control the temperature at 140°C and continue heating for 4 hours to obtain a brown-yellow solution. Pour the reaction solution into a beaker containing 250 mL of crushed ice and ice water. A large number of crystals precipitated in the lower part of the beaker. After stirring for 4 hours, let it stand, pour off the upper liquid, wash the crystals twice with ice water, filter, collect the crystals, and dry them to white The crude product was 10.84 g. The product was dissolved in absolute ethanol and refluxed for 1 hour, cooled, placed in ice water to precipitate crystals, filtered and dried. Obtained 8.67 grams of dry white prismatic crystal sample. The yield is 80.00...
Embodiment 3
[0029] Weigh 4.00 g of anhydrous sodium acetate and add it to a 100 mL round-bottomed three-neck flask, then add 25.00 mL of acetic anhydride, and stir. Heat to reflux with an oil bath, after the solution becomes clear, add a total of 5.00 g of anhydrous D-glucose within 30 minutes, then control the temperature of the oil bath at 135°C and continue heating for 3 hours to obtain a brownish yellow solution. Pour the reaction solution into a beaker filled with 250mL of ice water. A large amount of crystals precipitated in the lower part of the beaker. After stirring for 3 hours, let it stand, pour off the upper solution, wash the crystals twice with ice water, filter, collect the crystals, and dry to obtain a white crude product. 10.83 grams, the product was dissolved in absolute ethanol and refluxed for 0.5h, cooled, placed in ice water to precipitate crystals, filtered and dried. 8.23 g of the target product were obtained with a yield of 75.93% and a melting point of 131.2-131....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com