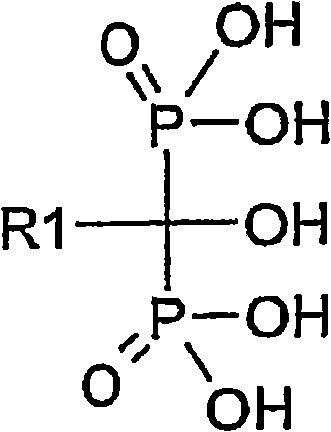
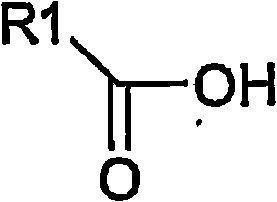
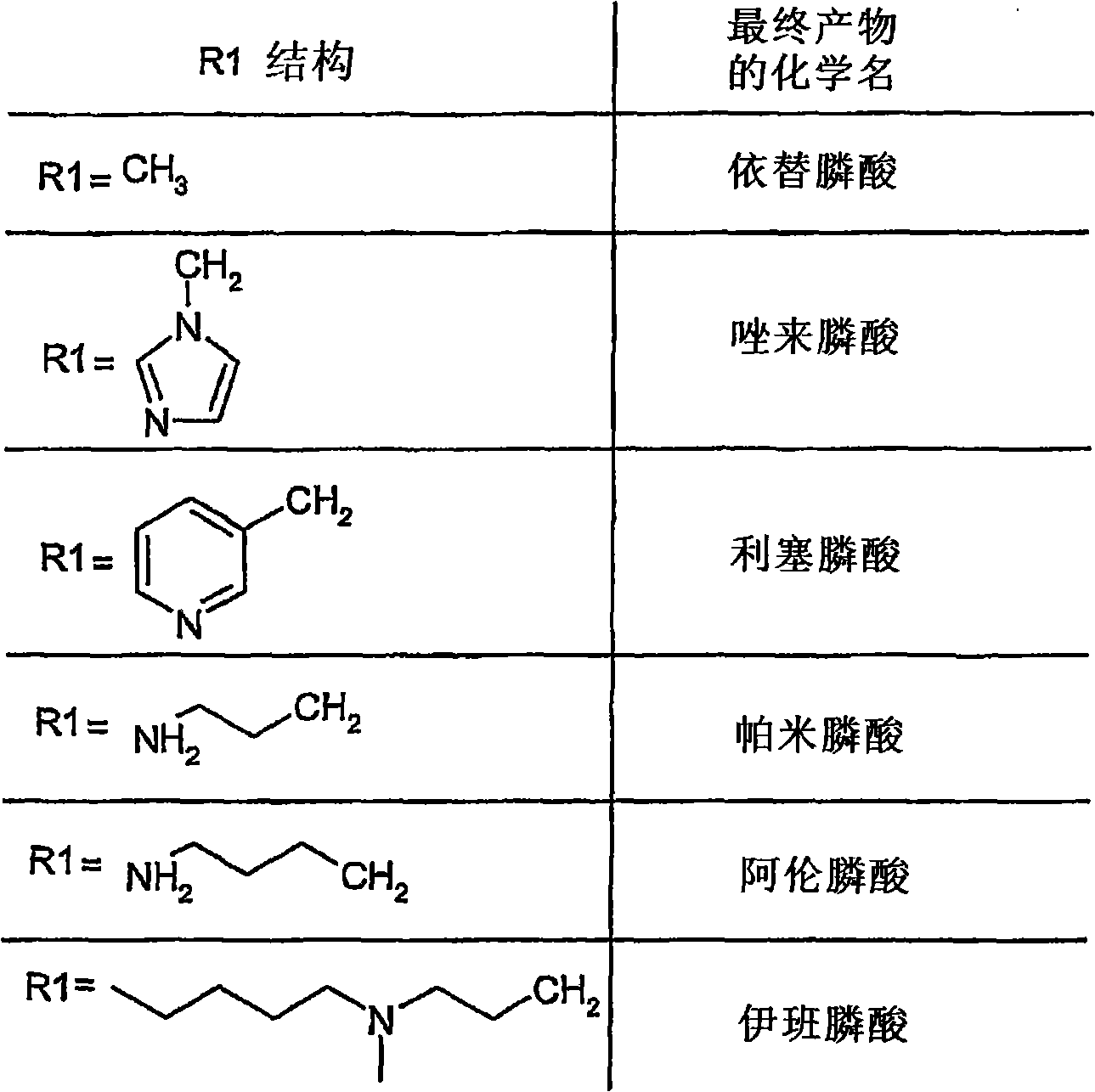
Process for the preparation of biphosphonic acids and salts thereof
A technology for bisphosphonic acid compounds and carboxylic acids, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc. Yield-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation of risedronate sodium salt:
[0059] 3-pyridineacetic acid (7.5g; 0.0432mol) and H 3 PO 3 (5.31 g; 0.0648 mol) in N,N'-dimethylethylene urea (DMEU) (30 ml) was heated to a temperature of 40°C to 50°C. PCl 3 (7.5ml; 0.0852mol) was slowly added to the resulting suspension. The resulting mixture was heated to a temperature of 50°C to 60°C and stirred until the reaction was complete. Reaction completion was monitored by HPLC. Water was slowly added to the reaction mixture, and the resulting solution was heated with stirring at a temperature of 80°C to 100°C until the reaction was complete. The reaction mixture was cooled to room temperature and the pH was adjusted to about pH 8-9 with aqueous sodium hydroxide. The resulting solution was filtered and the pH of the solution was adjusted to 4.5 to 5.0. Ethanol was added and a solid precipitated. The solid was filtered, washed, and dried under vacuum at a temperature of 45°C to 55°C to constant weight. 8.9 ...
Embodiment 2
[0065] Preparation of risedronic acid free acid:
[0066] 3-pyridineacetic acid (25g; 0.142mol) and H 3 PO 3 (17.7 g; 0.216 mol) in N,N'-dimethylethylene urea (DMEU) (100 ml) was heated to a temperature of 40°C to 50°C. PCl 3 (25.2ml; 0.284mol) was slowly added to the resulting suspension. The resulting mixture was heated to a temperature of 50°C to 60°C and stirred until the reaction was complete. Reaction completion was monitored by HPLC. Water was slowly added to the reaction mixture, and the resulting solution was heated with stirring at a temperature of 80°C to 100°C until the reaction was complete. The reaction mixture was cooled to room temperature and the pH was adjusted to about pH 8-9 with aqueous sodium hydroxide. The resulting solution was filtered, the pH of the solution was adjusted to 1.5 to 2.0, ethanol was added, and a solid precipitated. The solid was filtered, washed, and dried under vacuum at a temperature of 45°C to 55°C to constant weight.
[0067...
Embodiment 3
[0070] Preparation of risedronic acid free acid crude product:
[0071] 3-pyridineacetic acid (7.5g; 0.0432mol)) and H 3 PO 3 (5.31 g; 0.0648 mol) in N,N'-dimethylethylene urea (DMEU) (30 ml) was heated to a temperature between 40°C and 50°C. PCl 3 (7.5ml; 0.0852mol) was slowly added to the resulting suspension. The resulting mixture was heated to a temperature of 50°C to 60°C and stirred until the reaction was complete (by HPLC). Water is slowly added to the reaction mixture, and the resulting solution is heated to a temperature of 80°C to 100°C with stirring until the reaction is complete. The reaction mixture was cooled to room temperature. The solid was filtered, washed, and dried under vacuum at a temperature of 45°C to 55°C to constant weight. 12.9 g of crude risedronic acid were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com