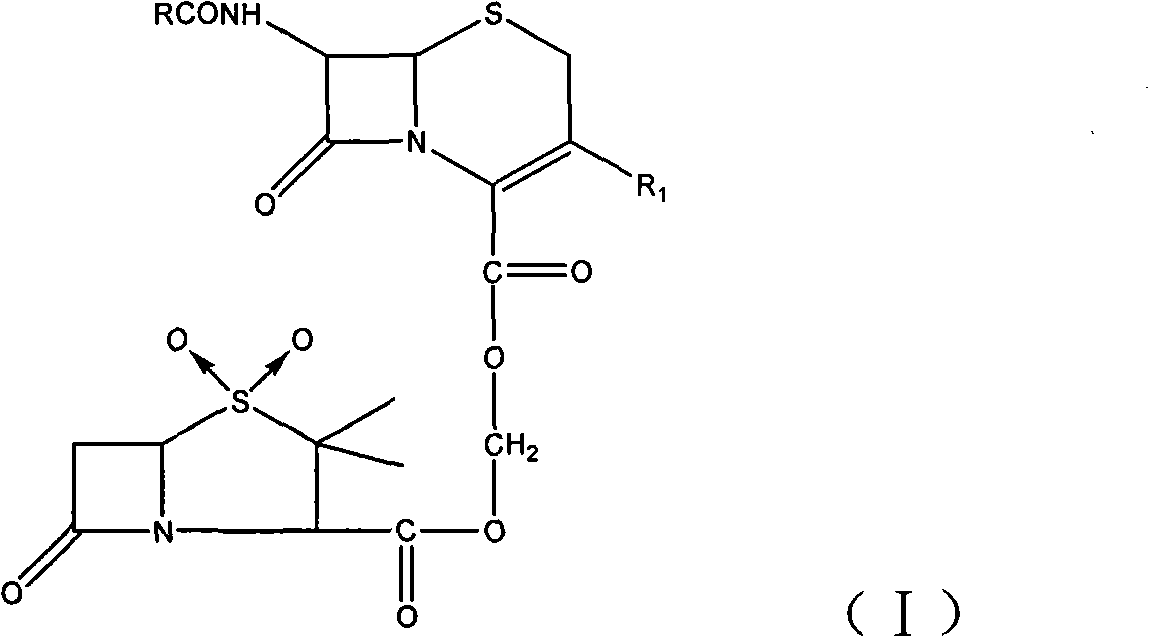
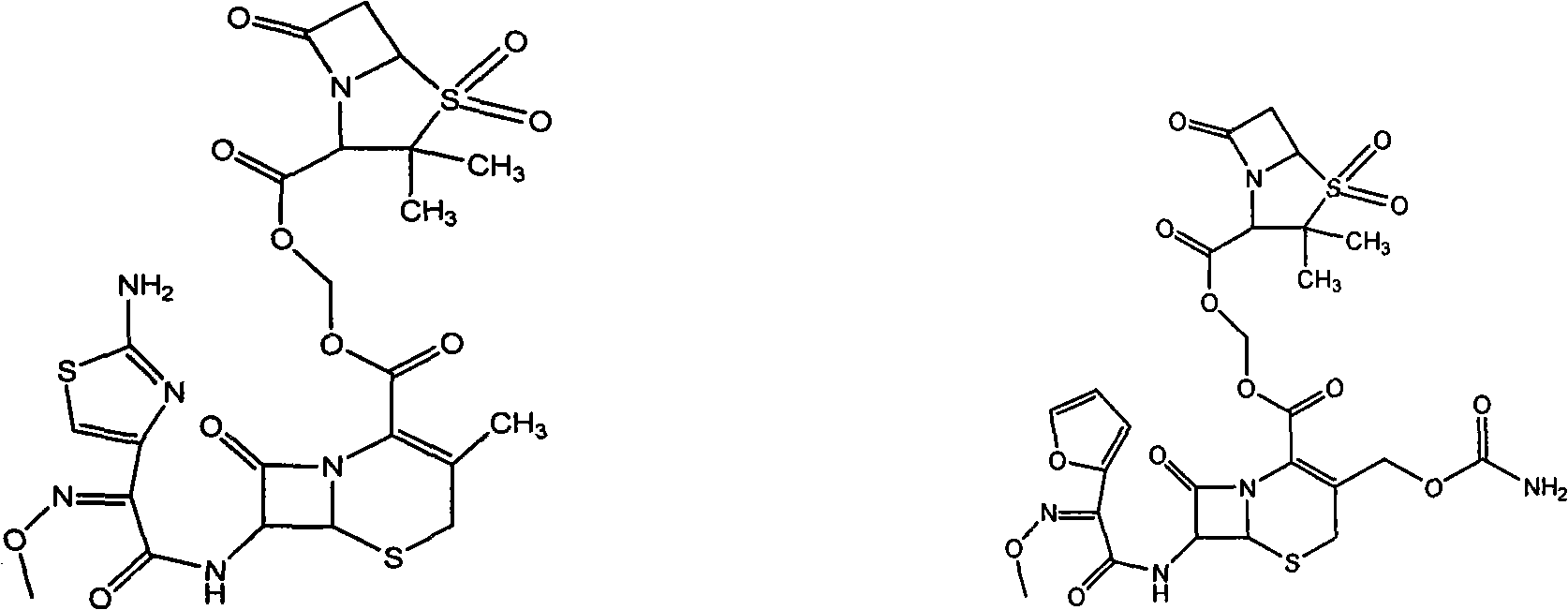
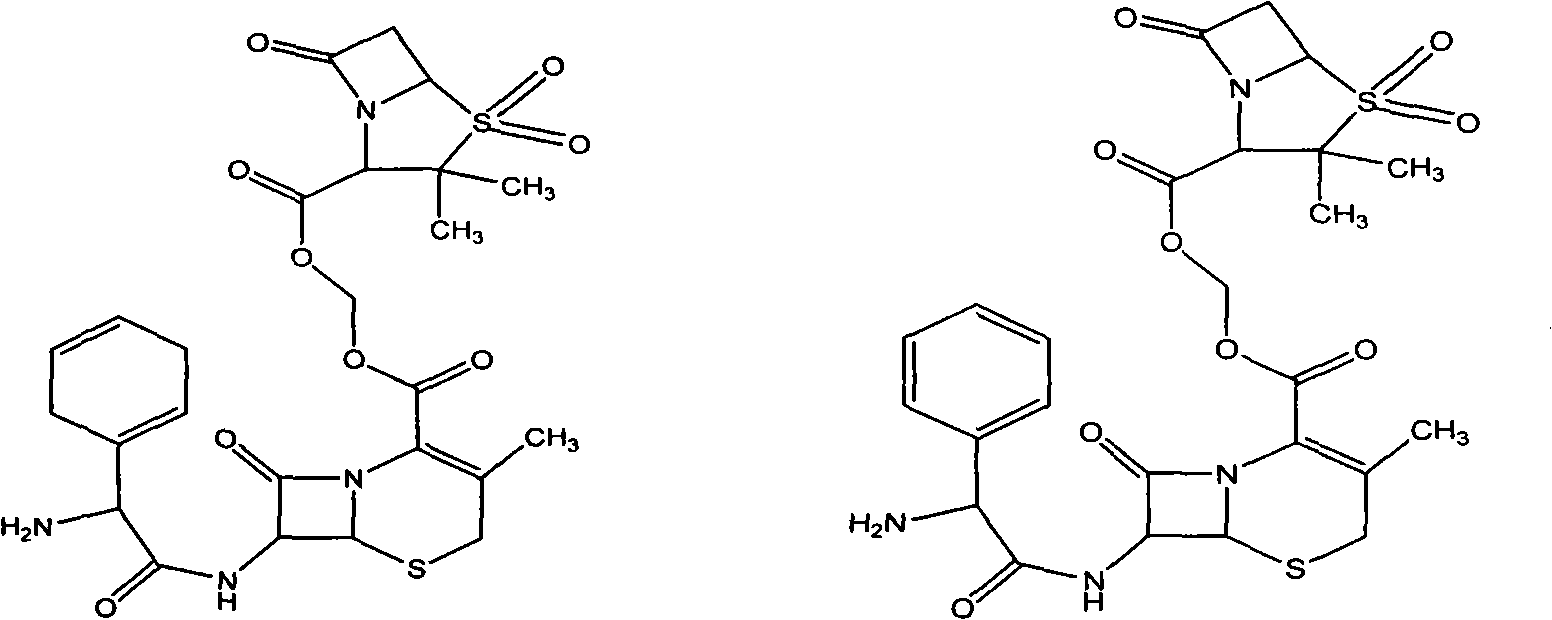
Sulphur butyl ether-beta-schardinger dextrin inclusion compound of cephalosporin sulbactam ester
A technology of inclusion compound of cephalosporin sulbactam mexetil and cyclodextrin, which is applied in the field of medicine and can solve problems such as not involving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 0.643 g of ceftamethoxil to 40 ml of an aqueous solution containing 8.8 g of sulfobutyl ether-β-cyclodextrin, stir, sonicate, dissolve, filter, and freeze-dry to obtain white ceftamet sulbactam Ester sulfobutyl ether-β-cyclodextrin inclusion compound powder.
[0031] Preparation of powder injection:
[0032] 500 g of the clathrate powder (calculated as ceftaxime) was aseptically divided into 1000 or 500 tubes to obtain ceftamet sulbactam medoxomil powder injections of different specifications.
Embodiment 2
[0034] Add 0.67 g of cefuroxime sulbactam axetil to 45 ml of an aqueous solution containing 11 g of sulfobutyl ether-β-cyclodextrin, stir, sonicate, dissolve, filter, and freeze-dry to obtain white cefuroxime sulbactam axetil The sulfobutyl ether-β-cyclodextrin inclusion compound powder.
[0035] Powder injection preparation
[0036] 500 g of the clathrate powder (calculated as cefuroxime) was aseptically divided into 2000 or 500 or 250 tubes to obtain cefuroxime sulbactam mexetil powder injections of different specifications.
Embodiment 3
[0038] Add 0.6 g of cephradine sulbactam medoxotil to 50 ml of an aqueous solution containing 13.3 g of sulfobutyl ether-β-cyclodextrin, stir, sonicate, filter, concentrate, and freeze-dry to obtain white sulfobutamic acid cephradine sulbactam mexetil Ether-β-cyclodextrin inclusion compound powder.
[0039] Powder injection preparation
[0040] Above-mentioned clathrate powder (calculated as cephradine) 1000g, aseptically sub-packed into 2000 or 1000, to obtain cephradine-sulbactam medoxomil powder injections of different specifications.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com