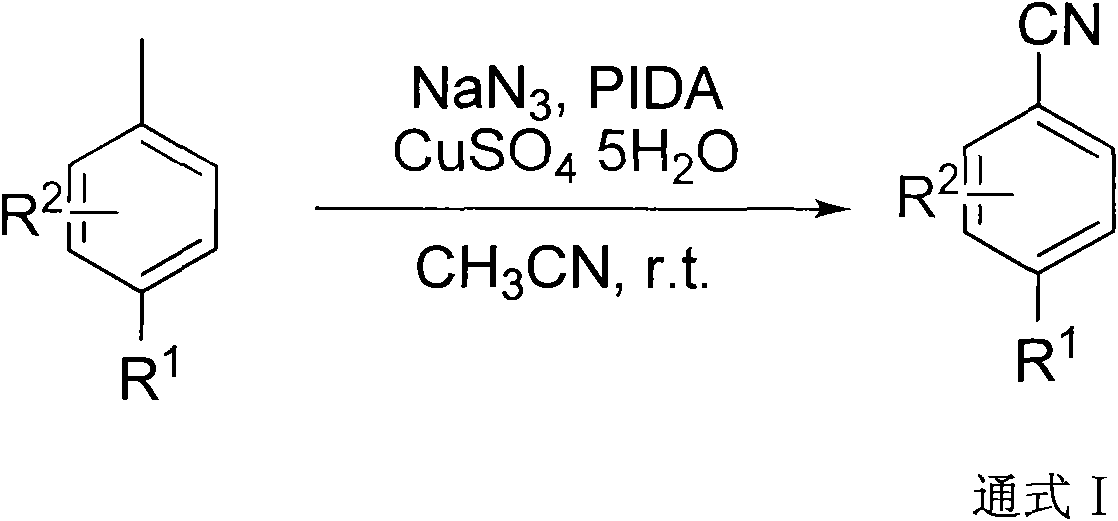Aromatic nitrile compounds or derivatives of same and synthetic method and application thereof
A compound, aryl nitrile technology, applied in the synthesis of aryl nitrile compounds or their derivatives, in the field of aryl nitrile compounds or their derivatives, can solve the problem of poor tolerance of substrate functional groups, not many, complicated operation, etc. problem, to achieve the effect of simple conditions, easy-to-obtain raw materials, and simple reaction equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 4-methoxybenzonitrile
[0027] Take a reaction tube, add 6.2 mg of copper sulfate, 64 mg of 4-methoxytoluene, 44 mg of sodium azide, 175 mg of iodobenzene acetate, and 4 ml of acetonitrile under the protection of nitrogen, and stir at room temperature for two hours. Sodium nitride (44mg×2) and iodobenzene acetate (175mg×2) were added every two hours. After the reaction was completed, it was filtered, the solvent was removed, and 47mg of pure product was obtained by column chromatography with a yield of 71%.
[0028] IR: (KBr)ν max 2969, 2924, 2854, 2219, 1606, 1510, 1461, 1260, 1176, 1024, 832cm -1 ; 1 H NMR (CDCl 3 , 300MHz): δ=7.51(d, J=8.9Hz, 2H), 6.87(m, J=8.9Hz, 2H), 3.78(s, 3H); 13 C NMR (CDCl 3 , 75MHz): δ=162.8, 133.9, 119.2, 114.7, 103.9, 55.5ppm; MS (70eV): m / z (%): 133.1 (M + , 100).
Embodiment 24
[0029] The preparation of embodiment 24-methoxybenzonitrile
[0030] Take a reaction tube, add 6.2 mg of copper sulfate, 64 mg of 4-methoxytoluene, 130 mg of sodium azide, 515 mg of iodobenzene acetate, and 4 ml of acetonitrile under nitrogen protection, and stir at room temperature. After the reaction is completed, filter and remove the solvent , 34 mg of pure product was obtained by column chromatography with a yield of 53%.
[0031] IR: (KBr)ν max 2969, 2924, 2854, 2219, 1606, 1510, 1461, 1260, 1176, 1024, 832cm -1 ; 1 H NMR (CDCl 3 , 300MHz): δ=7.51(d, J=8.9Hz, 2H), 6.87(m, J=8.9Hz, 2H), 3.78(s, 3H); 13 C NMR (CDCl 3 , 75MHz): δ=162.8, 133.9, 119.2, 114.7, 103.9, 55.5ppm; MS (70eV): m / z (%): 133.1 (M + , 100).
Embodiment 3
[0032] The preparation of embodiment 3 4-ethoxybenzonitrile
[0033] Take a reaction tube, add 6.2 mg of copper sulfate, 69 mg of 4-ethoxytoluene, 44 mg of sodium azide, 175 mg of iodobenzene acetate, and 4 ml of acetonitrile under nitrogen protection, and stir at room temperature for two hours, and then stack the other two Sodium nitride (44mg×2) and iodobenzene acetate (175mg×2) were added every two hours. After the reaction was completed, it was filtered, the solvent was removed, and 48mg of pure product was obtained by column chromatography with a yield of 65%.
[0034] IR: (KBr)ν max 2981, 2220, 1605, 1507, 1260, 1172, 1039, 845, 808cm -1 ; 1 H NMR (CDCl 3 , 300MHz): δ=7.57(d, J=9.2Hz, 2H), 6.93(d, J=9.2Hz, 2H), 4.08(q, J=7.0Hz, 2H), 1.44(t, J=7.0Hz ,3H); 13 CNMR (CDCl 3, 75MHz): δ=162.2, 133.9, 119.3, 115.1, 103.6, 63.9, 14.5ppm; HRMSm / z (ESI) calcd for C 9 h 9 NO(M+H) + : 148.0757, found 148.0753.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap