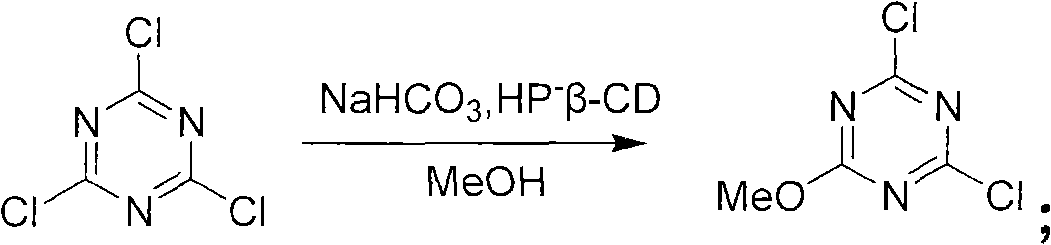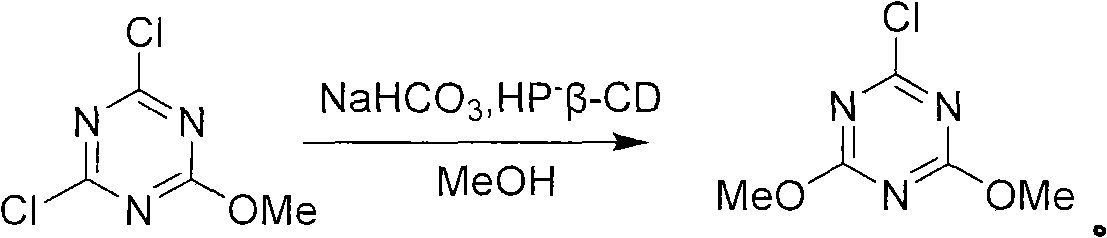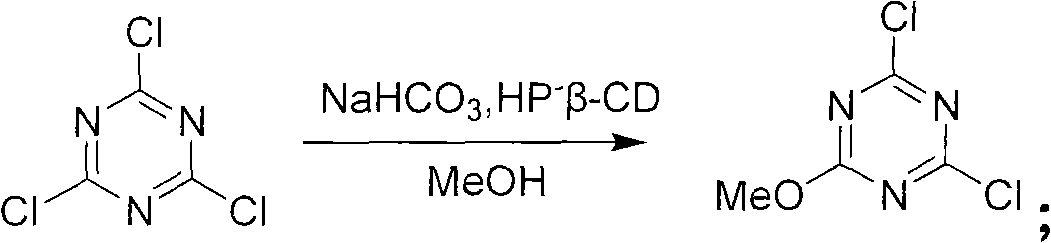Method for preparing 2-chlorine-4, 6-dimethoxy-1, 3, 5-triazine
A dimethoxyl, disubstituted technology, applied in the field of peptide coupling reagents, can solve the problems of high content of by-products, unsuitable for industrial production, etc., and achieve the effect of accelerating the reaction, increasing the yield, and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: A preparation method of 2-chloro-4,6-dimethoxy-1,3,5-triazine
[0025] Materials used: cyanuric chloride: 240kg; hydroxypropyl-β-cyclodextrin (HP-β-CD): 18Kg; methanol: 600kg; sodium bicarbonate: 327kg; water: 100kg; petroleum ether: 600kg
[0026] Process flow: (1) Synthesis reaction: The 2000L reactor is clean, the tail gas absorption device is in place, and the thermometer and other indicators are normal. Add sodium bicarbonate: 327kg, water: 100kg, catalytic amount of catalyst HP-β-CD 18Kg to the reaction kettle, then dissolve 240kg cyanuric chloride in 600Kg methanol, stir to completely dissolve the cyanuric chloride. Slowly add the methanol solution of cyanuric chloride dropwise to the reaction system at (25-30° C.), and control the reaction temperature to be 25-30° C. throughout the dropping process. At this time, a large number of bubbles are generated in the reaction system (slowly dropwise, control the reaction temperature so as not to react too vi...
Embodiment 2
[0029] Example 2: A preparation method of 2-chloro-4,6-dimethoxy-1,3,5-triazine
[0030] A preparation method of 2-chloro-4,6-dimethoxy-1,3,5-triazine, comprising the following steps:
[0031] The first step: mixing sodium bicarbonate, water and catalytic amount of catalyst hydroxypropyl-β-cyclodextrin evenly to obtain a reaction solution, wherein the mass ratio of water to sodium bicarbonate is 1:9;
[0032] The second step: under the condition of 10-15°C, slowly dropwise add the methanol solution dissolved in cyanuric chloride to the reaction solution obtained in the first step to generate monosubstituted triazine, the reaction equation is as follows:
[0033]
[0034] Wherein, the mass ratio of cyanuric chloride and methanol is 0.35: 1, and the mass ratio of cyanuric chloride and sodium bicarbonate is 0.9: 1;
[0035] Step 3: Raise the temperature to 65°C to continue the reaction of the monosubstituted triazine generated in the third step to generate a disubstituted tri...
Embodiment 3
[0039] Example 3: A preparation method of 2-chloro-4,6-dimethoxy-1,3,5-triazine
[0040] A preparation method of 2-chloro-4,6-dimethoxy-1,3,5-triazine, comprising the following steps:
[0041] The first step: mixing sodium bicarbonate, water and catalytic amount of catalyst hydroxypropyl-β-cyclodextrin evenly to obtain a reaction solution, wherein the mass ratio of water to sodium bicarbonate is 1:4;
[0042] The second step: under the condition of 20° C., slowly dropwise add a methanol solution dissolved in cyanuric chloride to the reaction solution obtained in the first step to generate a monosubstituted triazine;
[0043] Wherein, the mass ratio of cyanuric chloride and methanol is 0.6: 1, and the mass ratio of cyanuric chloride and sodium bicarbonate is 0.7: 1;
[0044] The third step: raising the temperature to 40° C. to continue the reaction of the mono-substituted triazine generated in the third step to generate a di-substituted triazine to obtain a mixture containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com