Method for synthesizing 1,4-dioxane-2-ketone by ethylene glycol
A technology of dioxane and ethylene glycol, applied in organic chemistry and other directions, can solve the problems of low industrial feasibility, large solvent usage, unfriendly environment, etc., and achieves strong industrial feasibility, simplified synthesis process, Avoiding the effect of ester by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
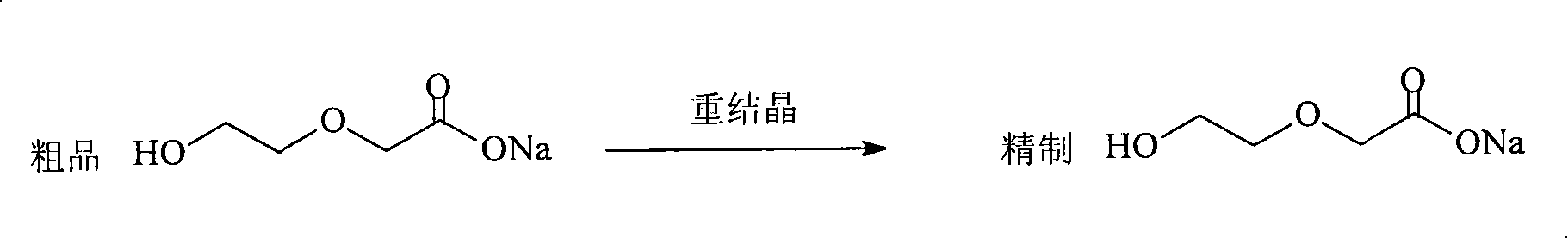
[0020] Example 1: Combining Figure 5 , the present invention synthesizes 1,4-dioxane-2-ketone with ethylene glycol, and saves the refining process of β-hydroxyethoxy sodium acetate, namely saves image 3 The purification steps shown. The process is as follows.
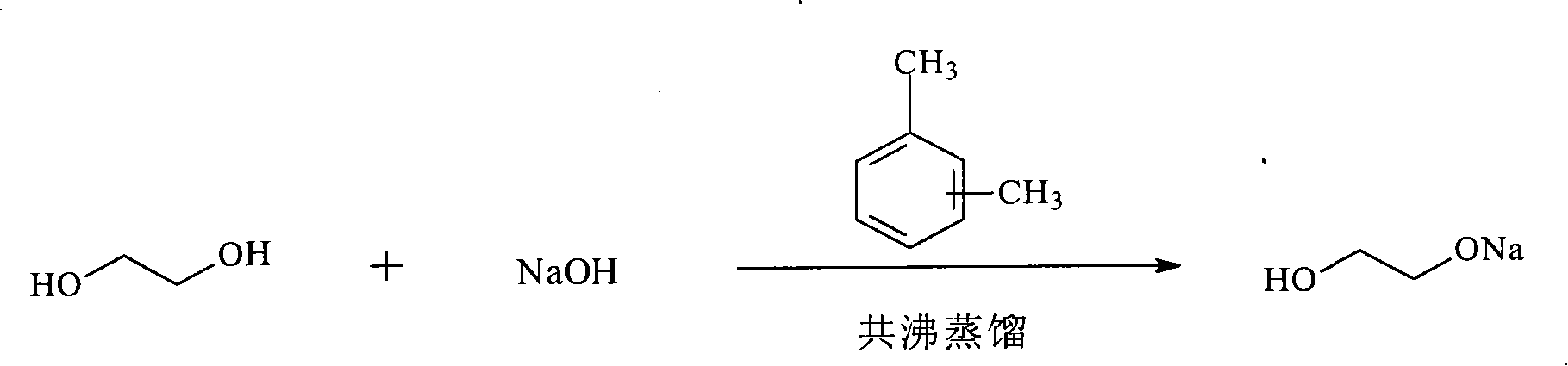
[0021] In the first step, add 10g of sodium hydroxide to a four-necked flask filled with 100ml of xylene and 50ml of ethylene glycol, slowly raise the temperature to 70°C~80°C, stir and dissolve into a light yellow liquid, and continue to heat up to 126°C~ 128°C, azeotropic distillation, reaction for 12h, the color of the solution changes as follows: colorless-light yellow-yellow-yellow brown. After the reaction is over, let the layers stand and pour out the supernatant, which is a mixture of ethylene glycol and xylene, and apply mechanically in the next cycle, such as figure 1 shown.
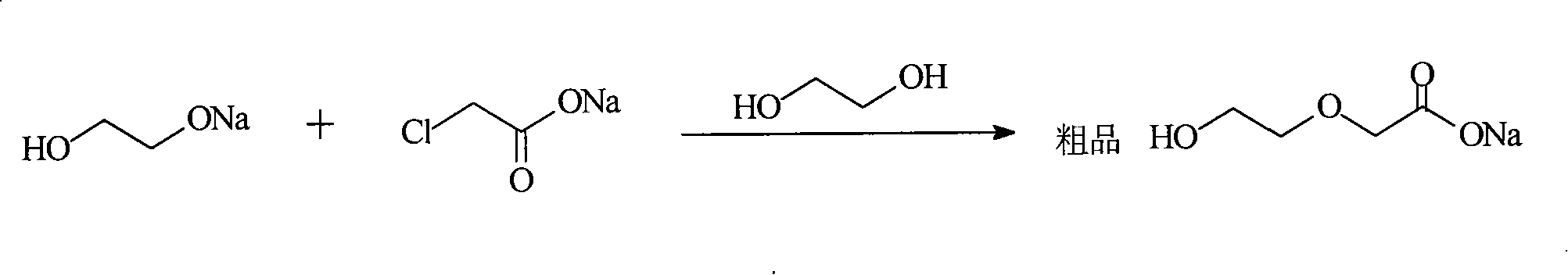
[0022] In the second step, add 50ml of ethylene glycol to the remaining solid in the first step reaction (a mixture of monosodium...
Embodiment 2
[0025] Embodiment 2: The present invention uses ethylene glycol to synthesize 1,4-dioxane-2-one, which saves the refining process of β-hydroxyethoxy sodium acetate, and the process is as follows.
[0026] In the first step, add 10g of sodium hydroxide to a four-necked flask filled with 100ml of toluene and 60ml of ethylene glycol, slowly heat up to 70°C-80°C, stir and dissolve into a light yellow liquid, and continue to heat up to 124°C-126°C °C, azeotropic distillation, reaction for 10h, the color of the solution changes as follows: colorless-light yellow-yellow-yellow brown. After the reaction finishes, let stand to separate layers, pour out the supernatant liquid, be the mixture of ethylene glycol and toluene, use mechanically and mechanically in the next cycle, such as figure 1 shown.
[0027] In the second step, add 50ml of ethylene glycol to the remaining solid in the first step reaction (a mixture of monosodium ethylene glycol and a small amount of ethylene glycol), sl...
Embodiment 3
[0030] Embodiment 3: The present invention uses ethylene glycol to synthesize 1,4-dioxane-2-one, which saves the refining process of β-hydroxyethoxy sodium acetate, and the process is as follows.
[0031] In the first step, add 10g of sodium hydroxide to a four-neck flask containing 100ml of benzene and 70ml of ethylene glycol, slowly heat up to 70°C-80°C, stir and dissolve into a light yellow liquid, and continue to heat up to 120°C-122 °C, azeotropic distillation, reaction for 8 hours, the color of the solution changes as follows: colorless-light yellow-yellow-tan. After the reaction finishes, let stand to separate layers, pour out the supernatant liquid, be the mixture of ethylene glycol and benzene, apply mechanically in the next cycle, such as figure 1 shown.
[0032] In the second step, add 50ml of ethylene glycol to the remaining solid in the first step reaction (a mixture of monosodium ethylene glycol and a small amount of ethylene glycol), slowly raise the temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com