Method for estimating residual capacity of iron-lithium phosphate power cell
A power battery and lithium iron phosphate technology, which is applied in the direction of measuring electrical variables, measuring electricity, and measuring devices, can solve the problems that the estimation method is not easy to satisfy, and achieve the effects of simple algorithm, reduced usage, and small cumulative error
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
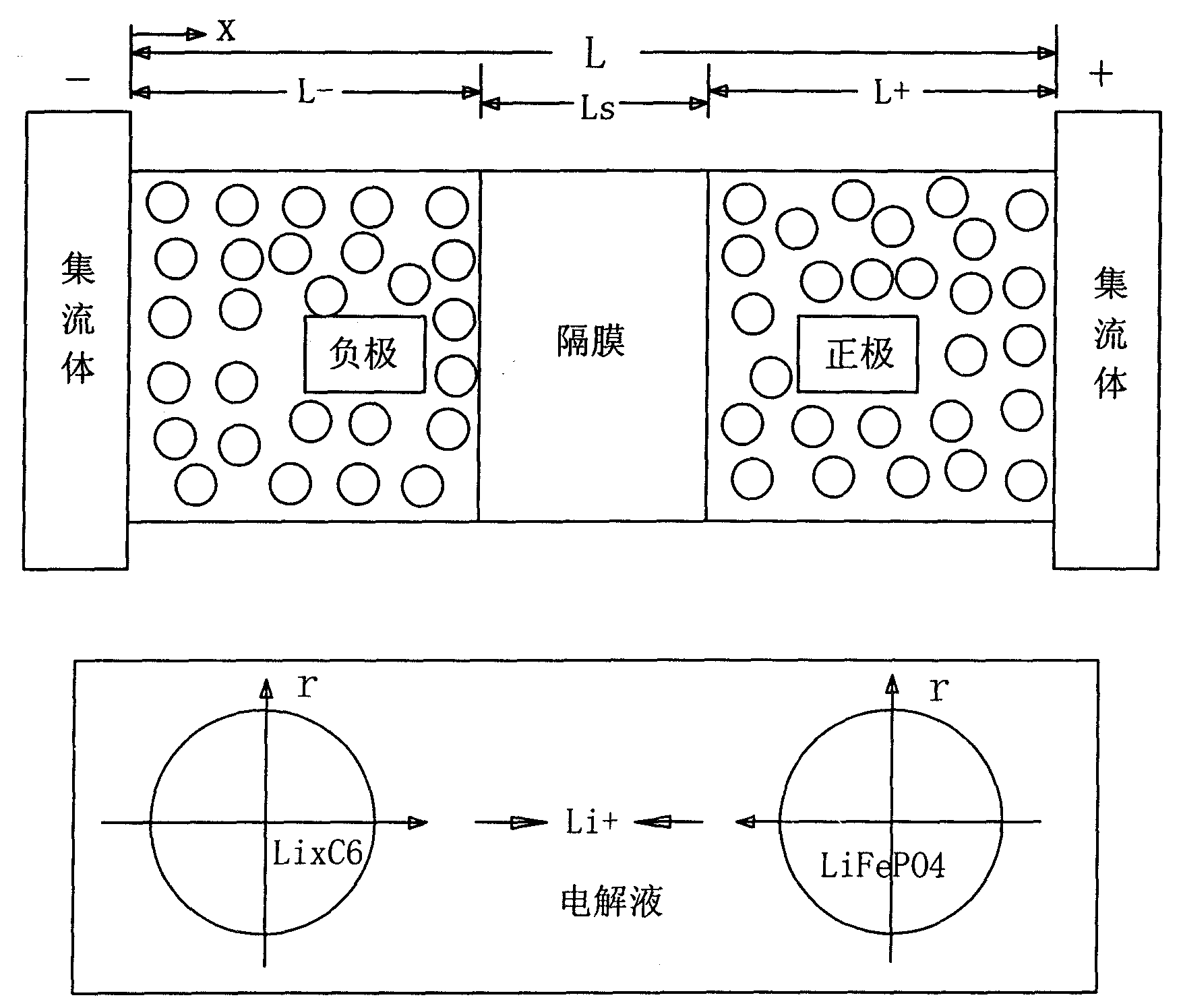
[0023] A method for estimating the remaining capacity of a lithium iron phosphate power battery, comprising the steps of:
[0024] 1. Establish the electrochemical model of the lithium iron phosphate power battery [the battery electrochemical model sees the (6) formula of the embodiment];
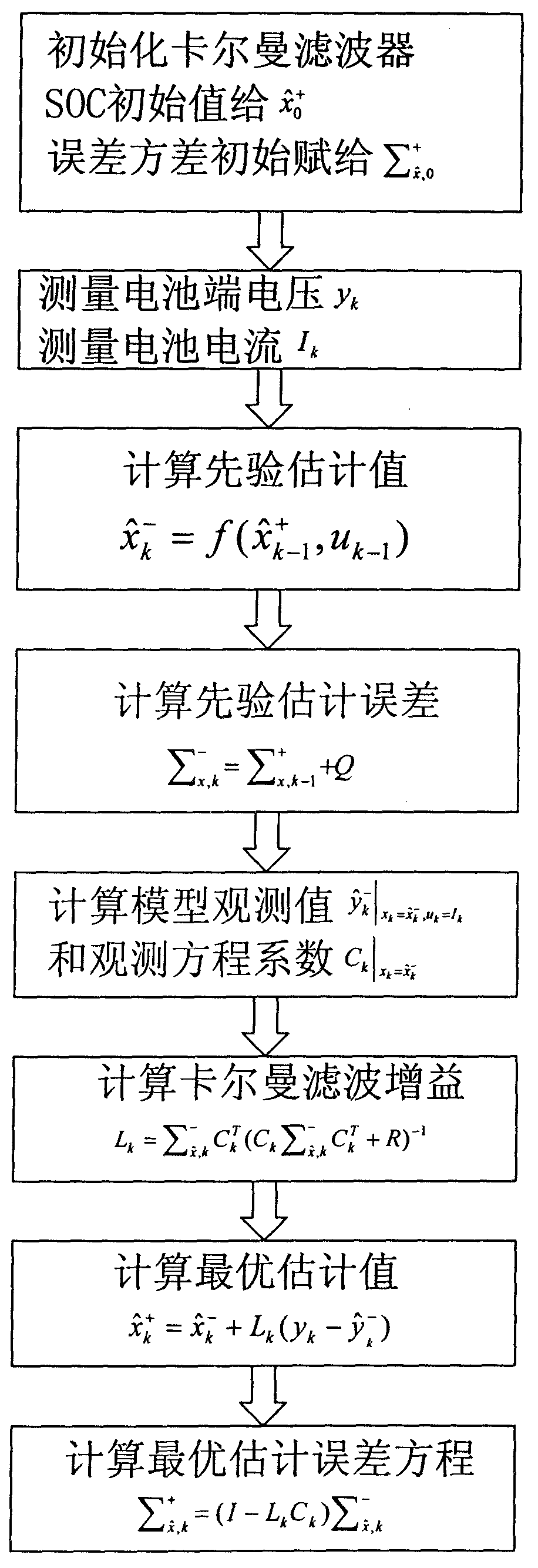
[0025] 2. According to the voltage equation of the electrochemical model, use the Kalman filter algorithm to obtain the remaining battery capacity:
[0026] Battery remaining capacity = the ratio of the average concentration of solid-phase lithium ions in the negative electrode of the battery to the maximum concentration of lithium ions when fully charged;
[0027] Among them, the solid-phase lithium ion concentration in the negative electrode of the battery is obtained through the extended Kalman filter algorithm; the maximum concentration of lithium ions during full charge is the calibration value at the factory, which is a constant.
[0028] In order to match the electrochemical model o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com