Use of Semaphorin 6A for Promoting Myelination and Oligodendrocyte Differentiation
A technology of oligodendrocytes and myelin sheaths, applied in the fields of neurology, pharmacology, and neurobiology, which can solve problems such as poorly understood functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
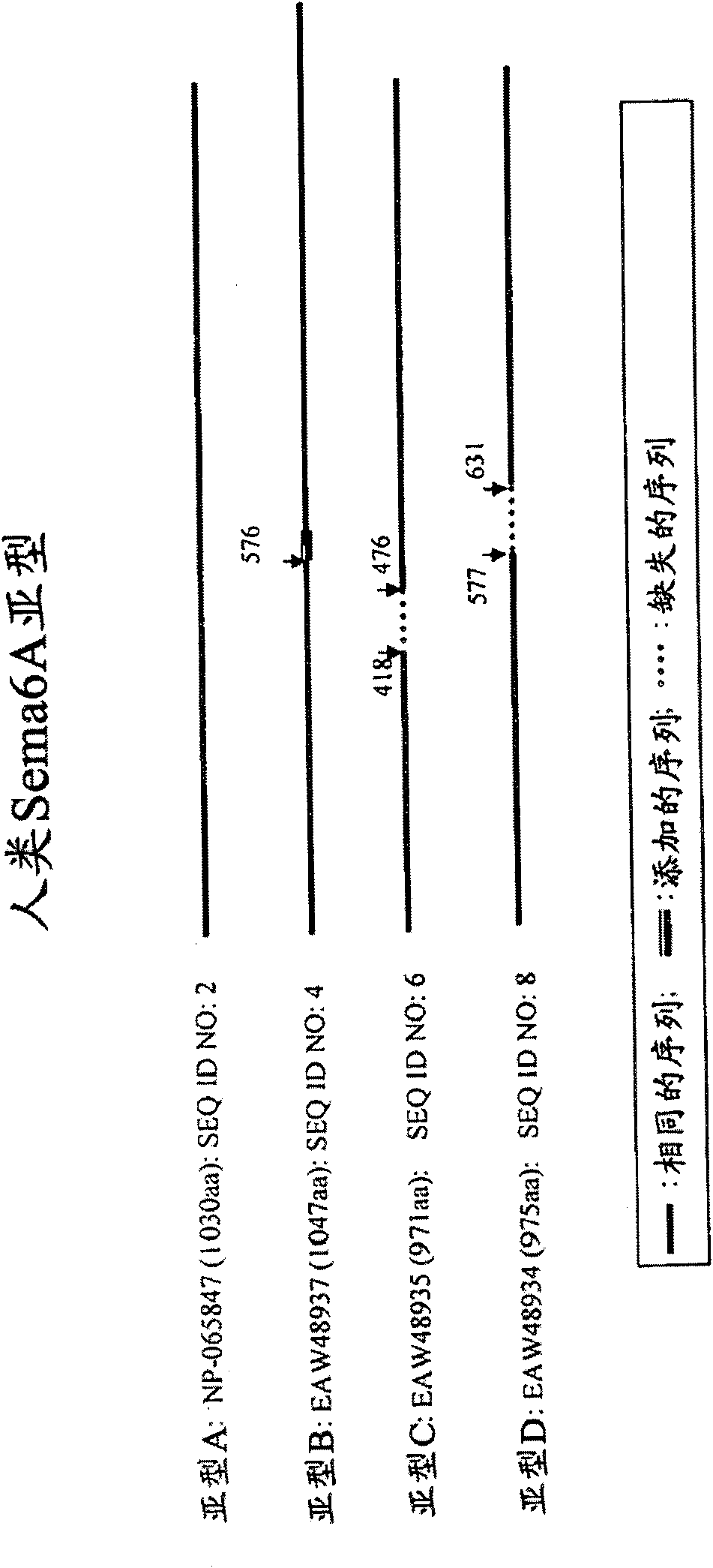
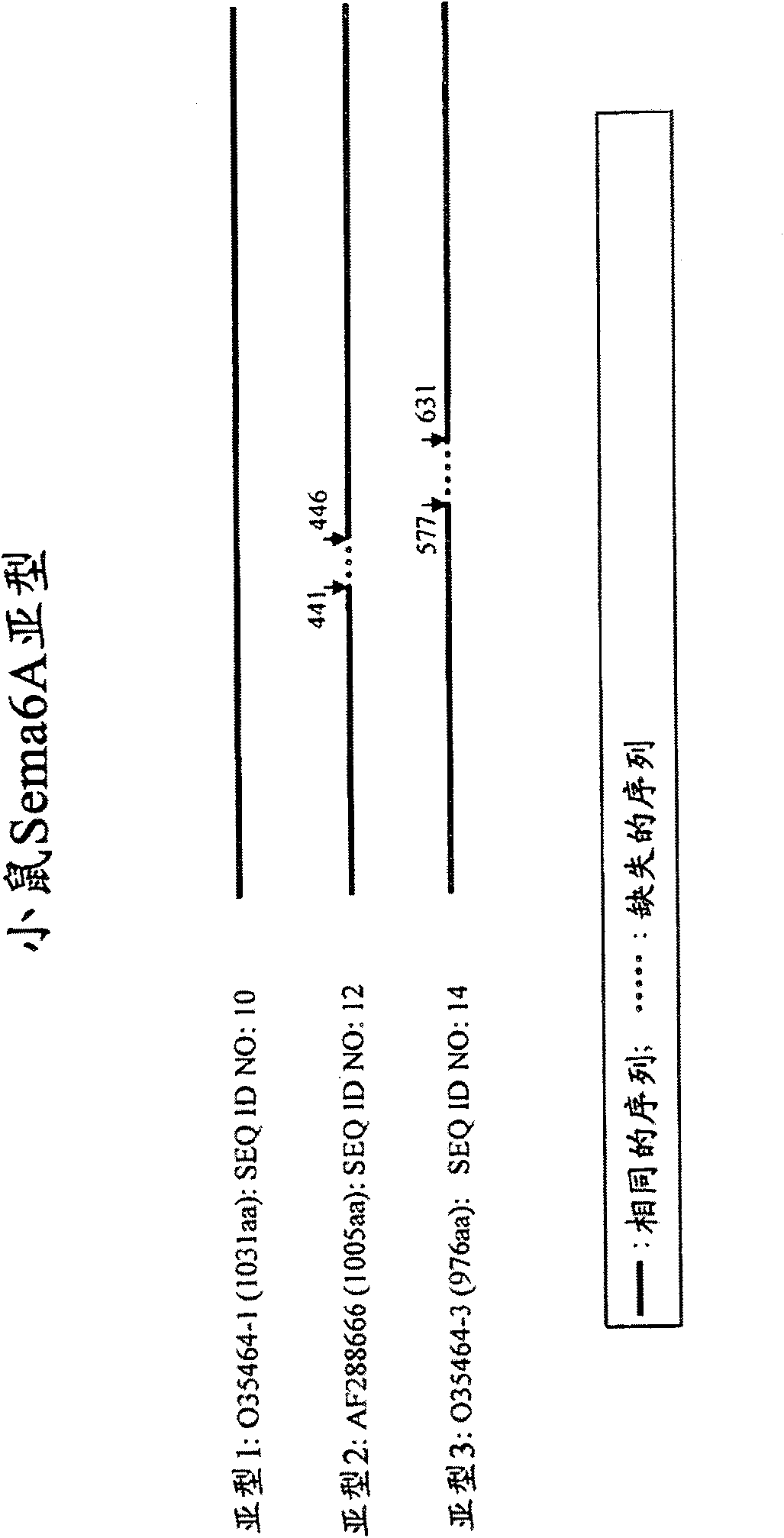
[0213] Sema6A is involved in the biology of oligodendrocytes
[0214]Oligodendrocyte maturation goes through several developmental stages, from oligodendrocyte progenitor cells (which express NG2) to pre-myelinating oligodendrocytes (which express O1 and O4 ) and eventually differentiate into mature myelinating oligodendrocytes (which express O1, O4, myelin basic protein (MBP) and anti-proteolipid protein (PLP)). Thus, by monitoring the presence or absence of NG2, O1, O4, MBP and PLP markers, it is possible to determine the developmental stage of a given cell and evaluate the role of Sema6A polypeptides in oligodendrocyte biology. Oligodendrocyte transcription factor-2 (Olig-2) is also thought to be expressed in the oligodendrocyte lineage and is therefore used as a marker to detect oligodendrocytes. See Yokoo et al., Amer. J. of Path. 164:1717-1725 (2004) (for a review of oligodendrocyte biology see, eg, Baumann and Pham-Dinh, Physiol. Rev. 81:871-927 (2001) ; Bras et al., ...
Embodiment 2
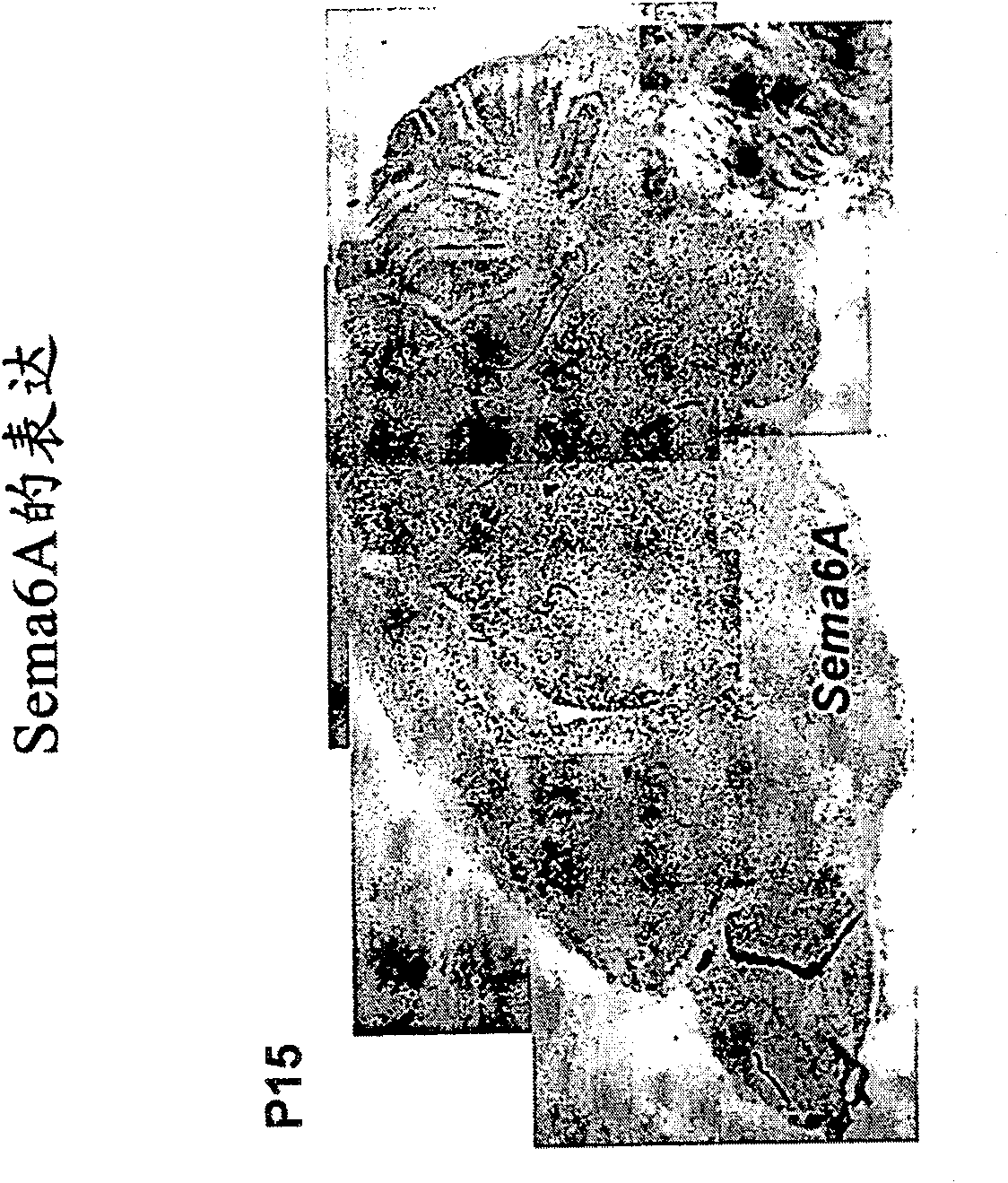
[0225] Expression of Sema6A in different stages of oligodendrocyte differentiation
[0226] In vitro Sema6A expression was also shown in purified oligodendrocyte cultures. The cortical hemispheres of P0 to P5 mice were excised and transferred to a medium consisting of DMEM supplemented with 10% calf serum. In culture medium, the tissue was separated by passing through a 70 μm mesh nylon mesh. The cell suspension was dispensed onto 100 mm diameter plastic tissue culture dishes coated with polyornithine. Oligodendrocyte precursor cells were selectively isolated by gently syringeing the medium above the cell layer. The removed cells were then subjected to two consecutive preplatings on uncoated plastic dishes over a 12 hour period to allow the remaining astrocytes and microglia to attach. Non-attached as well as loosely attached cells (oligodendrocytes) were subcultured on 60mm plastic dishes. Anti-mouse Sema6A antibody and anti-NG2 (marker of oligodendrocyte progenitor cells...
Embodiment 3
[0228] Sema6A-knockout mice exhibit reduced myelinated axons
[0229] To generate Sema6A-knockout mice, the transmembrane domain encoding CD4-galactosidase-neomycin phosphotransferase (TM--geo) separated by an internal ribosome entry site (IRES) and human The cassette for placental alkaline phosphatase (PLAP) was inserted into intron 17 of Sema6A as described in Leighton et al., Nature, 410:174-179. The remaining N-terminal portion of the Sema6A protein up to amino acid 623 (thus lacking the transmembrane and cytoplasmic domains) is fused to -galactosidase and retained in the endoplasmic reticulum.
[0230] To analyze the delay in myelination, the nodes of Ranvier in Sema6A-deficient mice were studied. The junction of Ranvier expresses a variety of proteins whose expression and function have been demonstrated to be characteristic of the junction. Antibodies raised against proteins involved in the formation of Ranvier junctions, such as paranodin, were used to detect protein ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com