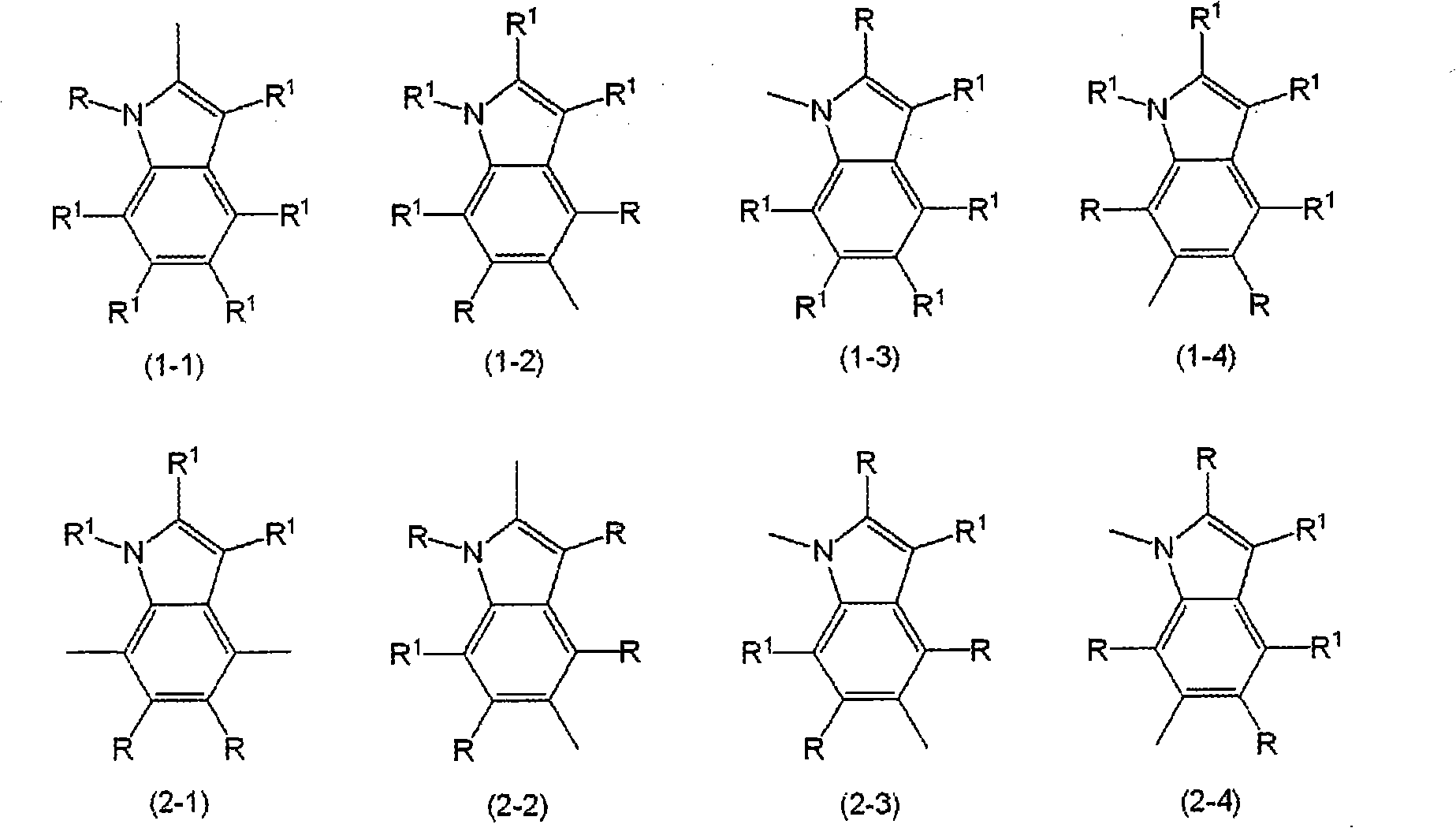
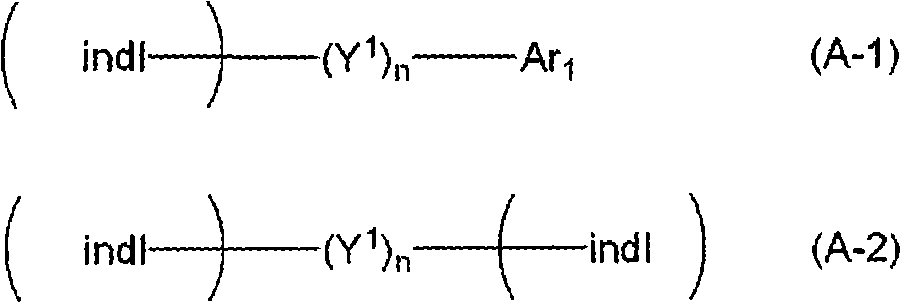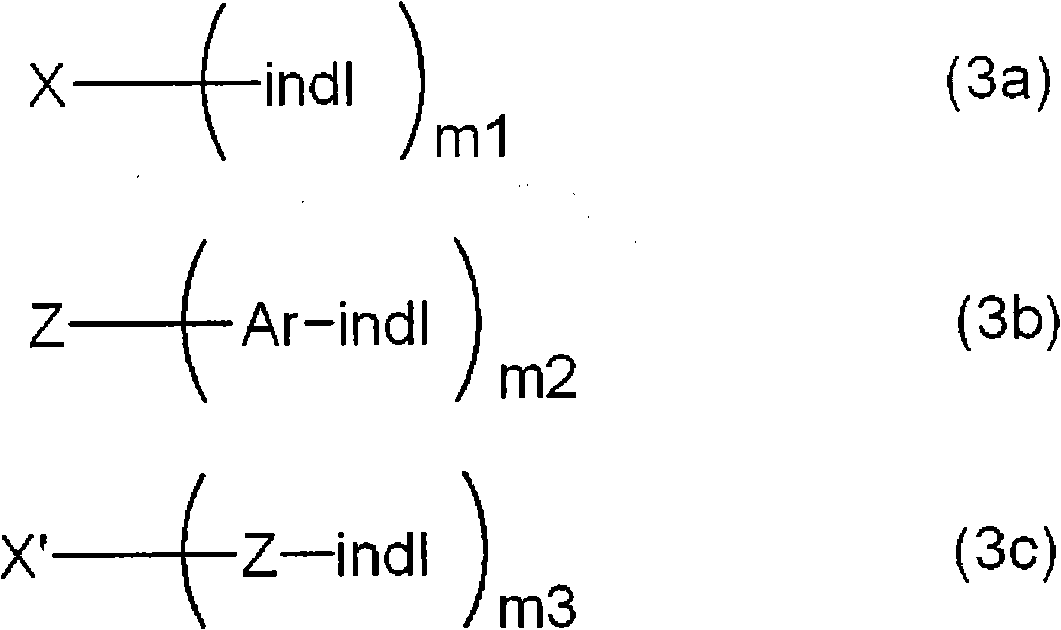Indole compound-containing composition and light-emitting device using the composition
A technology for light-emitting elements and compounds, applied in the field of compositions containing indole compounds, can solve the problems of low luminous efficiency and low triplet excitation energy of light-emitting materials, and achieve excellent luminous efficiency, large triplet excitation energy and low LUMO. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] The minimum triplet excitation energy T of the polymer (P-1) represented by the following formula, where n=∞ is an extrapolated value 1 (1 / n=0) is 3.1eV, the absolute value of the energy level E of the lowest unoccupied molecular orbital energy level LUMO (1 / n=0) is 1.4eV, and the minimum dihedral angle is 46°.
[0147]
[0148] (In the formula, n is the aggregation number.)
[0149] Calculation of the parameters is carried out using computational science methods described in the detailed description of the invention. Specifically, using the following repeating unit (M-1) in the polymer (P-1), for the cases of n=1, 2, and 3, the structure was optimized by the F method.
[0150]
[0151] At this time, as the basis function, use 6-31G * . Then, using the same basis function, the energy level of the lowest unoccupied molecular orbital energy level and the lowest triplet excitation energy were calculated through the time-dependent density functional method of the ...
Embodiment 2
[0155] With respect to the THF solution (0.05% by weight) of the phosphorescent compound (MC-1) represented by the following formula (MC-1), the THF solution (about 5 times the weight) of the compound represented by the following formula (C-1) was mixed. 1% by weight). 10 µl of the resulting solution was dropped on a slide glass and air-dried to obtain a solid film. When it was irradiated with ultraviolet rays of 365 nm, green light emission from the phosphorescent compound (MC-1) was confirmed.
[0156]
[0157] The lowest triplet excitation energy T of the compound represented by the above formula (C-1) 1 is 3.3eV, the absolute value of the energy level E of the lowest non-occupied molecular orbital LUMO is 1.5eV.
[0158] It should be noted that the above formula (MC-1) was synthesized based on the method described in WO02 / 066552.
Embodiment 3
[0160] A THF solution (about 1% by weight) of a compound represented by the following formula (C-2) was mixed about 5 times by weight with respect to a THF solution (0.05% by weight) of the phosphorescent compound (MC-1). 10 µl of the resulting solution was dropped on a slide glass and air-dried to obtain a solid film. When it was irradiated with ultraviolet rays of 365 nm, green light emission from the phosphorescent compound (MC-1) was confirmed.
[0161]
[0162] The lowest triplet excitation energy T of the compound represented by the above formula (C-2) 1 is 3.3eV, the absolute value of the energy level E of the lowest unoccupied molecular orbital LUMO is 1.5eV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com