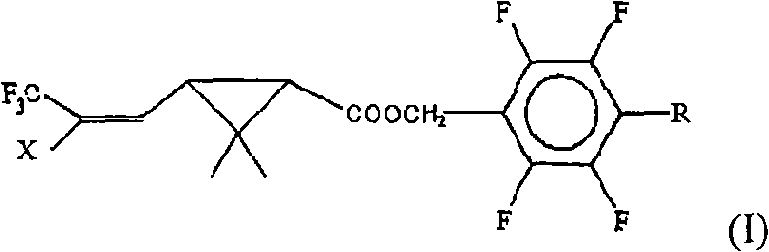
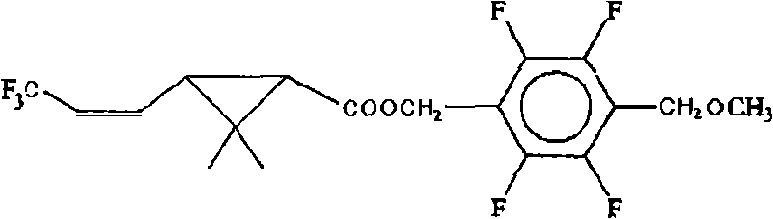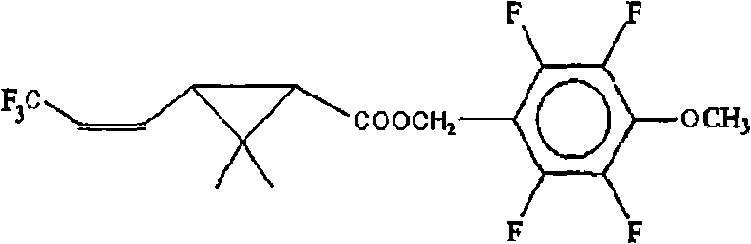Novel pyrethroid compounds derived from tefluthrin
A technology for pyrethroids and compounds, applied in the field of pyrethroids, can solve the problems of high toxicity and insecticidal efficacy, restricted use, long residual effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthesis of embodiment 1 compound (1)
[0026] 23.2 g of 3-(3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid chloride was added to the dropping funnel, and 110 g of toluene, Pyridine 9.5g, 2,3,5,6-tetrafluoro-4-methoxymethylbenzyl alcohol 22.9g, stir to dissolve completely, drop 3-(3,3,3- Trifluoro-1-propenyl)-2,2-dimethylcyclopropane carboxylic acid chloride, add dropwise for 1 hr, end the heat preservation and continue stirring for 0.5-1 hr. Add 5% hydrochloric acid solution for pickling, separate the water phase, and wash the oil layer with 5% NaHCO 3 The solution was washed with alkali, and then the oil layer was washed twice with deionized water. The washed oil layer removes toluene to obtain compound (1): 3-(3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid-2,3,5,6 -Tetrafluoro-4-methoxymethylbenzyl ester, the weight is 41.5g, the content is 95.2%, and the yield is 95.4%.
Embodiment 2
[0027] The synthesis of embodiment 2 compound (2)
[0028] 23.2 g of 3-(3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid chloride was added to the dropping funnel, and 110 g of toluene, Pyridine 9.5g, 2,3,5,6-tetrafluoro-4-methoxybenzyl alcohol 21.4g, stir to dissolve completely, drop 3-(3,3,3-tris Fluoro-1-propenyl)-2,2-dimethylcyclopropane carboxylic acid chloride, add dropwise for 1 hr, end the heat preservation and continue stirring for 0.5-1 hr. Add 5% hydrochloric acid solution for pickling, separate the water phase, and wash the oil layer with 5% NaHCO 3 The solution was washed with alkali, and then the oil layer was washed twice with deionized water. The washed oil layer removes toluene to obtain compound (1): 3-(3,3,3-trifluoro-1-propenyl)-2,-dimethylcyclopropanecarboxylic acid-2,3,5,6- Tetrafluoro-4-methoxybenzyl ester has a weight of 39.9 g, a content of 95.5%, and a yield of 95.2%.
Embodiment 3
[0029] The synthesis of embodiment 3 compound (3)
[0030] 23.2 g of 3-(3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid chloride was added to the dropping funnel, and 110 g of toluene, Pyridine 9.5g, 2,3,5,6-tetrafluoro-4-methylbenzyl alcohol 19.8g, stir to dissolve completely, drop 3-(3,3,3-trifluoro - 1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid chloride, add dropwise for 1 hr, end the heat preservation and continue stirring for 0.5-1 hr. Add 5% hydrochloric acid solution for pickling, separate the water phase, and wash the oil layer with 5% NaHCO 3 The solution was washed with alkali, and then the oil layer was washed twice with deionized water. The washed oil layer removes toluene to obtain compound (1): 3-(3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid-2,3,5,6 -Tetrafluoro-4-methylbenzyl ester, the weight is 38.2g, the content is 96.1%, and the yield is 95.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com