Pyrethroid compound, preparation method and application thereof
A technology of pyrethroids and compounds, which is applied in the field of pyrethroid compounds to achieve the effect of quickly killing pests
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
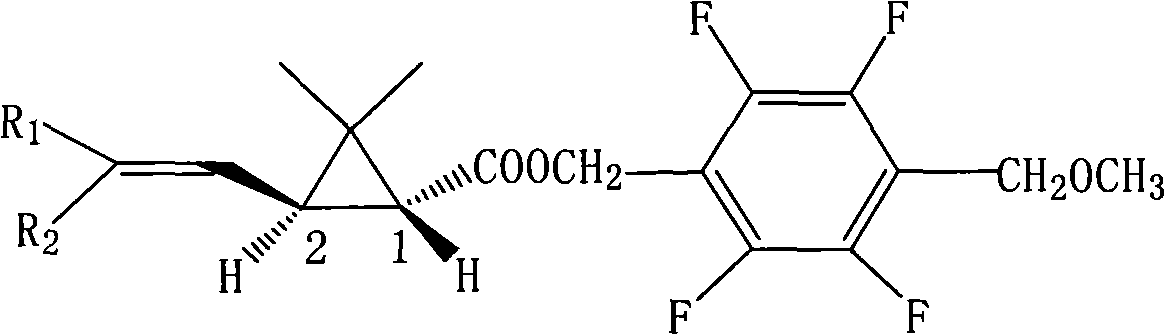
[0042] 2,3,5,6-Tetrafluoro-4-methoxymethylbenzyl (1R,3S)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate (Compound I) Synthesis:
[0043] In a 2000ml four-necked bottle, put 112.0g of tetrafluoro-p-methoxymethylbenzyl alcohol and 38.0g of pyridine, dissolve in 800ml of toluene, stir after throwing in, and add (R)-2,2 - 114.0 g of dimethyl-3-trans-(2,2-dichlorovinyl)cyclopropanecarboxylic acid chloride was added dropwise to 20°C for 4 hours. Wash with 400ml 5% hydrochloric acid, then wash with 400ml 5% NaHCO 3 After washing, the oil layer was heated to 100°C under a negative pressure of 10mmHg to remove the solvent toluene to obtain the compound 2,3,5,6-tetrafluoro-4-methoxymethylbenzyl (1R,3S)-3-(2,2 -dichlorovinyl)-2,2-dimethylcyclopropane carboxylate as light yellow solid, weight 203.3g, content 97.8%, yield 95.8%. The molecular formula of the compound: C 17 h 17 Cl 2 f 4 0 3 ; Molecular weight: 416.2; Optical rotation [α] = -9.32; Soluble in organic solven...
preparation Embodiment 2
[0045] 2,3,5,6-Tetrafluoro-4-methoxymethylbenzyl (1R,3S)-3-(2,2-dibromoethenyl)-2,2-dimethylcyclopropanecarboxylate Synthesis of (Compound II):
[0046] Put 56.0g of tetrafluoro-p-methoxymethylbenzyl alcohol, 25g of pyridine, and 400ml of toluene into a 1000ml four-necked bottle, stir for 15 minutes at room temperature, and drop (R)-2,2-dimethyl at 0-5°C Base-3-trans-(2,2-dibromovinyl)cyclopropanecarboxylic acid chloride 79.4g, after dripping, keep warm at 10°C for 1 hour, wash the oil layer with 150ml 5% hydrochloric acid, and then wash with 100ml 5% NaHCO 3 Wash, then wash until neutral. The oil layer was detoluene under negative pressure, the final temperature was 100°C, and the absolute pressure was 10mmHg to obtain the compound 2,3,5,6-tetrafluoro-4-methoxymethylbenzyl (1R,3S)-3-(2,2-di Bromovinyl)-2,2-dimethylcyclopropanecarboxylate is an off-white solid with a weight of 125.1 g, a content of 97.1%, and a yield of 96.2%.
preparation Embodiment 3
[0048] 2,3,5,6-tetrafluoro-4-methoxymethylbenzyl (1R,3S)-3-(2-chloro-2-bromovinyl)-2,2-dimethylcyclopropanecarboxylic acid Synthesis of ester (compound III):
[0049] Put 112.0g of tetrafluoro-p-methoxymethylbenzyl alcohol, 50.0g of pyridine, and 800ml of toluene into a 2000ml four-neck flask, stir to dissolve, and drop (R)-2-chloro-2-bromo- 136.2g of 3-trans-(2,2-dichlorovinyl)cyclopropanecarboxylic acid chloride, after the dropwise addition, keep the reaction at 10°C for 4 hours, pickle with 5% hydrochloric acid, and wash with 5% NaHCO 3 The solution was washed with alkali, and then the oil layer was washed twice with 200 ml of deionized water. The oil layer was detoluene under negative pressure, the final temperature was 100°C, and the absolute pressure was 10mmHg to obtain the compound 2,3,5,6-tetrafluoro-4-methoxymethylbenzyl (1R,3S)-3-(2-chloro 2- Bromovinyl)-2,2-dimethylcyclopropanecarboxylate is an off-white solid with a weight of 229.8g, a content of 98.2%, and a yi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com