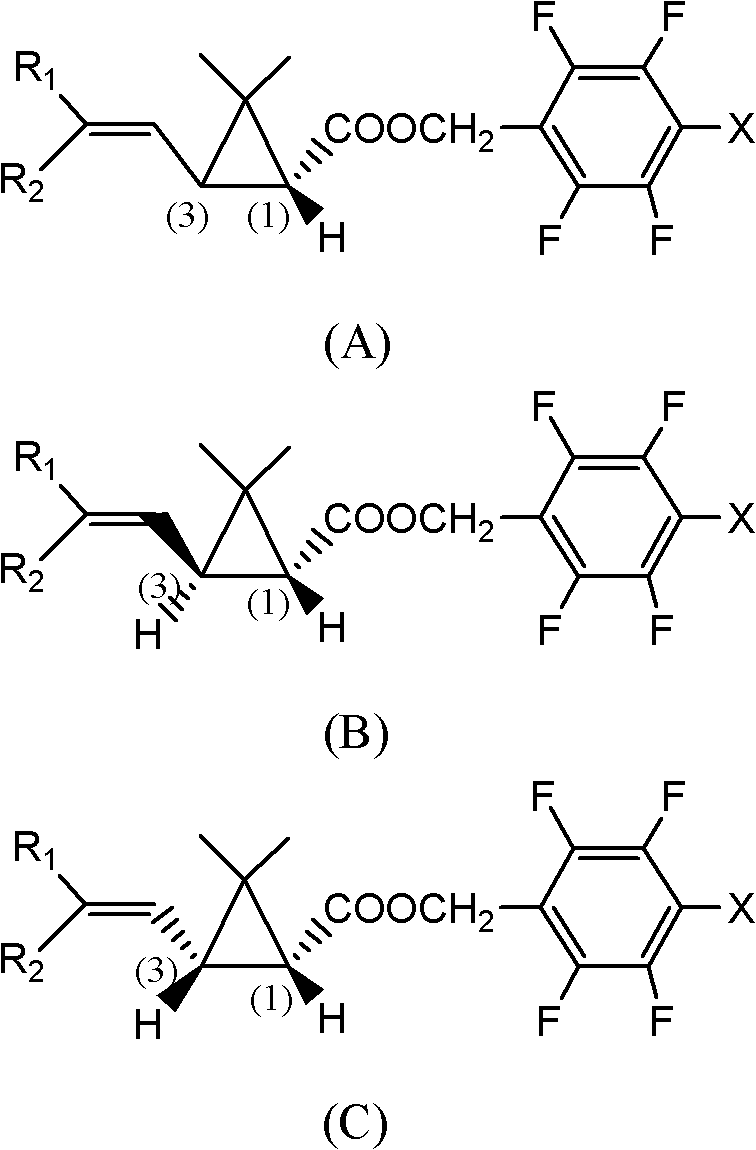
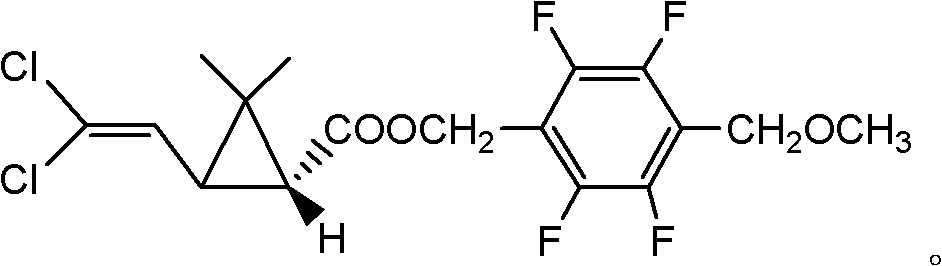
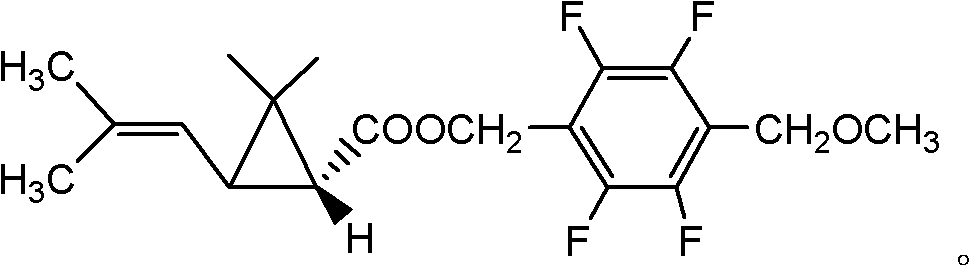
Optically active synthetic pyrethroid compound and preparation method and application thereof
A technology for pyrethroids and compounds is applied in the field of preparation of pyrethroids and can solve problems such as rising production costs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0068] A kind of preparation of chiral cyclopropane catalyst (chiral Cu(II) catalyst) of the present invention
[0069] Under the protection of argon, 0.30g (0.50mmol) (R)-1,1-bis[(2-n-octyloxy-5-tert-butyl)phenyl]-2-amino-1-propanol , 0.12g (0.50mmol) 2-bromo-4-nitrosalicylaldehyde, 0.11g (0.52mmol) copper acetate monohydrate were added to 10mL anhydrous methanol in sequence, after stirring at room temperature for half an hour, 0.0416g solid NaOH was added (1.04mmol), stirred overnight, evaporated the solvent, added 20mL water to the residue, extracted three times with 30mL benzene, and dried the organic phase with anhydrous potassium carbonate. The solvent was distilled off under reduced pressure to obtain 0.38 g of a chiral cyclopropane catalyst of the present invention——Cu(II) / Schiff base catalyst.
preparation Embodiment 2
[0071] Synthesis of compound I(a) (trans:compound I with a cis ratio of 60:40)
[0072] 1) Synthesis of cis and trans dextrorotatory 2,2-dimethyl-3-(2,2-dichlorovinyl)cyclopropanecarboxyethyl ester
[0073]In a 50ml four-neck flask, add 0.01mmol of the chiral cyclopropane catalyst prepared according to the method of Preparation Example 1, 1mmol of 2-methyl-5,5-dichloro-2,4-pentadiene and 2ml dichloroethane solvent, preheat to 80°C, slowly add 1.2mmol ethyl diazoacetate to the above reactant, specifically, a few drops can be added first to initiate the reaction, and the temperature is lowered to 75°C under the protection of argon. Then within 3 hours, use a syringe pump to add the remaining ethyl diazoacetate solution at a constant speed, continue to stir for 2 hours, and the reaction is over. The reaction solution is directly separated from 15 grams of silica gel to remove the cyclopropanation catalyst, and then washed with 20ml of dichloromethane. Silica gel was concentrated...
preparation Embodiment 3
[0077] Synthesis of Compound II
[0078] 1) Resolution of trans-2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylic acid
[0079] In a 1000ml four-necked bottle, put 90.0 g of trans-2,2-dimethyl-3-(2-methyl-1-propenyl) cyclopropanecarboxylic acid and 80.0 g of d-chloramphenicol, dissolve Add 500ml of toluene, stir after throwing in, heat up to 110°C and reflux for 1 hour, then cool to 40°C within 3 hours, keep warm for 1 hour, then cool to 10°C within 2 hours, keep warm for 0.5 hours, there are a lot of crystals at this time Precipitate. Filter, add 100g of 10% hydrochloric acid to the obtained mother liquor to acidify to PH=2-3, separate layers, wash the oil layer with water to near neutrality, heat to 100°C under 10mmHg negative pressure to remove solvent toluene, and obtain trans dextrorotary ( 1R,3R)-2,2-Dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylic acid 40.5g, dextrorotatory active body ee value 96%.
[0080] 2) Resolution of cis 2,2-dimethyl-3-(2-methy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com