New process for the synthesis of agomelatine
A technology of agomelatine and a synthetic method, which is applied in the field of industrially synthesized agomelatine or N-[2-ethyl]acetamide, can solve the problems of low average yield, achieve low cost, Controlling the Effect of Region Selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
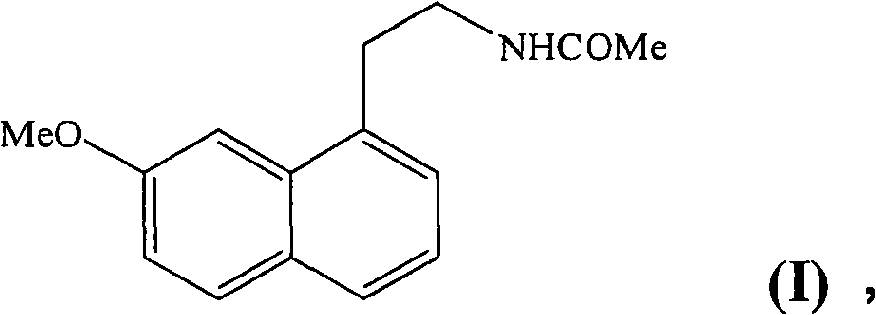
[0039] Example 1 :N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide
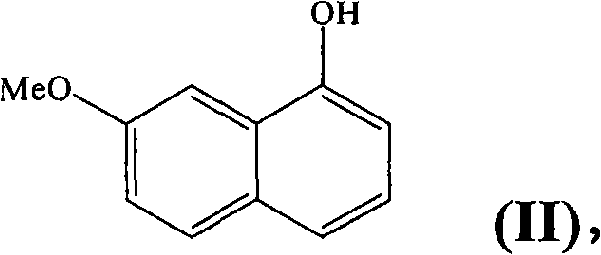
[0040] Step A :7-Methoxy-1-naphthyl Trifluoromethanesulfonate
[0041] In a reactor, 2.7 g of 7-methoxy-1-naphthol, 1.1 equivalents of trifluoromethanesulfonic anhydride and 1.1 equivalents of 2,6-di-tert-butyl-4-methyl-pyridine were introduced into the dichloride Methane (45ml). The mixture was heated under reflux for 12 hours, then it was filtered and the liquid was washed with 1N HCl solution and then with saturated NaCl solution. The organic phase was evaporated and the resulting residue was purified by silica gel chromatography (eluent: CH 2 Cl 2 / Methyl-cyclohexane 1 / 9) to obtain the title product with an oily chemical purity higher than 99% in a yield of 91%.
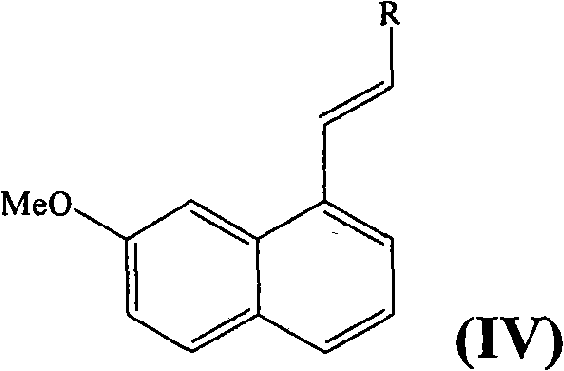
[0042] Step B :2-[2-(7-Methoxy-1-naphthyl)vinyl]-1H-isoindole-1,3(2H)-dione
[0043] In a reactor, 2g of the compound obtained in step A, 2 equivalents of N-vinylphthalimide, 1.25 equivalents of diisopropylethylamine and 0.05 equivalents of tetrakis(triph...
Embodiment 2
[0053] Example 2 :N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide
[0054] Step A :3-(7-methoxy-1-naphthyl)-2-acrylamide
[0055] The solution of the compound (12.1 g) obtained in Example 1 Step A in 80 mL DMF was degassed by bubbling with nitrogen gas at 20°C for 10 minutes. Add triethylamine (6.6mL), acrylamide (5.6g), neocopper hydrate (454mg) and Pd(OAc) to the resulting solution in sequence 2 (445mg). The mixture was heated at 100°C for 1 hour and then allowed to cool to 20°C. Dilute with AcOEt (100mL) and add saturated NH 4 After the Cl solution, the phases were separated. The organic phase was concentrated under reduced pressure, and the residue was diluted with AcOEt (50 mL). The precipitate was filtered off to obtain the title compound in powder form.
[0056] Step B :3-(7-Methylhydro-1-naphthyl)propionamide
[0057] 0.12 g of 5% Pd / C (50% humidity) was added to the solution of the compound (0.5 g) obtained in step A in a mixture of MeOH (6.5 mL) / THF (6.5 mL). The mixture ...
Embodiment 3
[0064] Example 3 : Determination of the crystal form of the compound N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide obtained in Examples 1 and 2
[0065] Data recording was performed with a D8 high-resolution diffractometer from Bruker AXS, using the following parameters: 3°-90° 2θ angle range, 0.01° span and 30 seconds for each span. The N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide powder obtained in Examples 1 and 2 was placed on a transfer fixture support. The X-ray source is a copper tube (λCuK α1 = 1.54056 ). The fixture includes a front monochromator (Ge(111) crystal) and an energy resolution solid-state detector (MXP-D1, Moxtec-SEPH). The compound crystallized well: the half-height line width was 0.07° (in 2θ).
[0066] The following parameters are determined accordingly:
[0067] -The crystal structure of the unit cell: monoclinic,
[0068] -Unit cell parameters: a=20.0903 b=9.3194 c=15.4796 β=108.667°
[0069] -Space group: P2 1 / n
[0070] -Number of molecules in the unit ce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com