New process for the synthesis of agomelatine
A technology of agomelatine and a synthetic method, which is applied in the field of industrially synthesized agomelatine or N-[2-ethyl]acetamide, can solve the problems of low average yield and the like, achieves low cost, Controls the effect of area selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
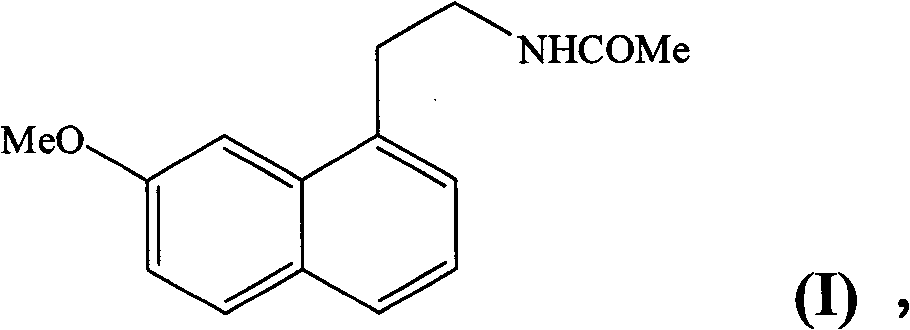
[0039] Example 1 : N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide
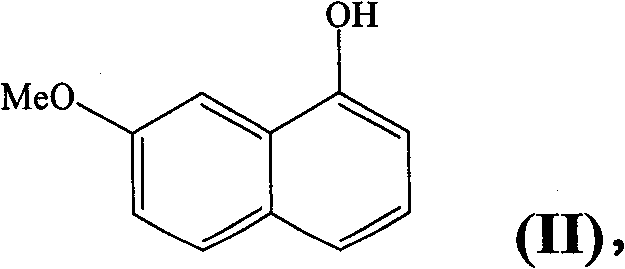
[0040] Step A : 7-methoxy-1-naphthyl trifluoromethanesulfonate
[0041] In one reactor, 2.7 g of 7-methoxy-1-naphthol, 1.1 equivalents of trifluoromethanesulfonic anhydride and 1.1 equivalents of 2,6-di-tert-butyl-4-methyl-pyridine were introduced into dichloro in methane (45ml). The mixture was heated at reflux for 12 hours, then it was filtered and the liquid was washed with 1N HCl solution, then with saturated NaCl solution. The organic phase was evaporated and the residue obtained was purified by silica gel chromatography (eluent: CH 2 Cl 2 / methyl-cyclohexane 1 / 9) to obtain the title product in 91% yield as an oil with a chemical purity higher than 99%.
[0042] Step B : 2-[2-(7-methoxy-1-naphthyl)vinyl]-1H-isoindole-1,3(2H)-dione
[0043] In one reactor, 2 g of the compound obtained in step A, 2 equivalents of N-vinylphthalimide, 1.25 equivalents of diisopropylethylamine and 0.05 equivalents of tet...
Embodiment 2
[0053] Example 2 : N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide
[0054] Step A : 3-(7-methoxy-1-naphthyl)-2-acrylamide
[0055] A solution of the compound obtained in Example 1, Step A (12.1 g) in 80 mL of DMF was degassed by bubbling nitrogen gas at 20°C for 10 minutes. To the resulting solution were sequentially added triethylamine (6.6 mL), acrylamide (5.6 g), neocopper reagent hydrate (454 mg) and Pd(OAc) 2 (445 mg). The mixture was heated at 100°C for 1 hour, then allowed to cool to 20°C. Dilute with AcOEt (100 mL) and add saturated NH 4 After the Cl solution, the phases were separated. The organic phase was concentrated under reduced pressure, and the residue was diluted with AcOEt (50 mL). The precipitate was filtered off to give the title compound as a powder.
[0056] Step B : 3-(7-methylhydrogen-1-naphthyl)propionamide
[0057] 0.12 g of 5% Pd / C (50% humidity) was added to a solution of the compound obtained in step A (0.5 g) in a mixture of MeOH (6.5 mL) / T...
Embodiment 3
[0064] Example 3 : the determination of embodiment 1 and 2 gained compound N-[2-(7-methoxy-1-naphthyl) ethyl] acetamide crystal form
[0065] Data recording was performed with a D8 high resolution diffractometer from Bruker AXS, using the following parameters: 2Θ angle range from 3° to 90°, in steps of 0.01° and 30 seconds per span. The N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide powder obtained in Examples 1 and 2 was placed on a transport fixture support. The X-ray source is a copper tube (λCuK α1 =1.54056 ). The fixture consisted of a pre-monochromator (Ge(111) crystal) and an energy-resolving solid-state detector (MXP-D1, Moxtec-SEPH). The compound crystallizes well: the linewidth at half maximum is 0.07° (in 2Θ).
[0066] The following parameters were determined accordingly:
[0067] - crystal structure of the unit cell: monoclinic,
[0068] -Unit cell parameter: a=20.0903 b=9.3194 c=15.4796 β=108.667°
[0069] - Space group: P2 1 / n
[0070] - Number of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com