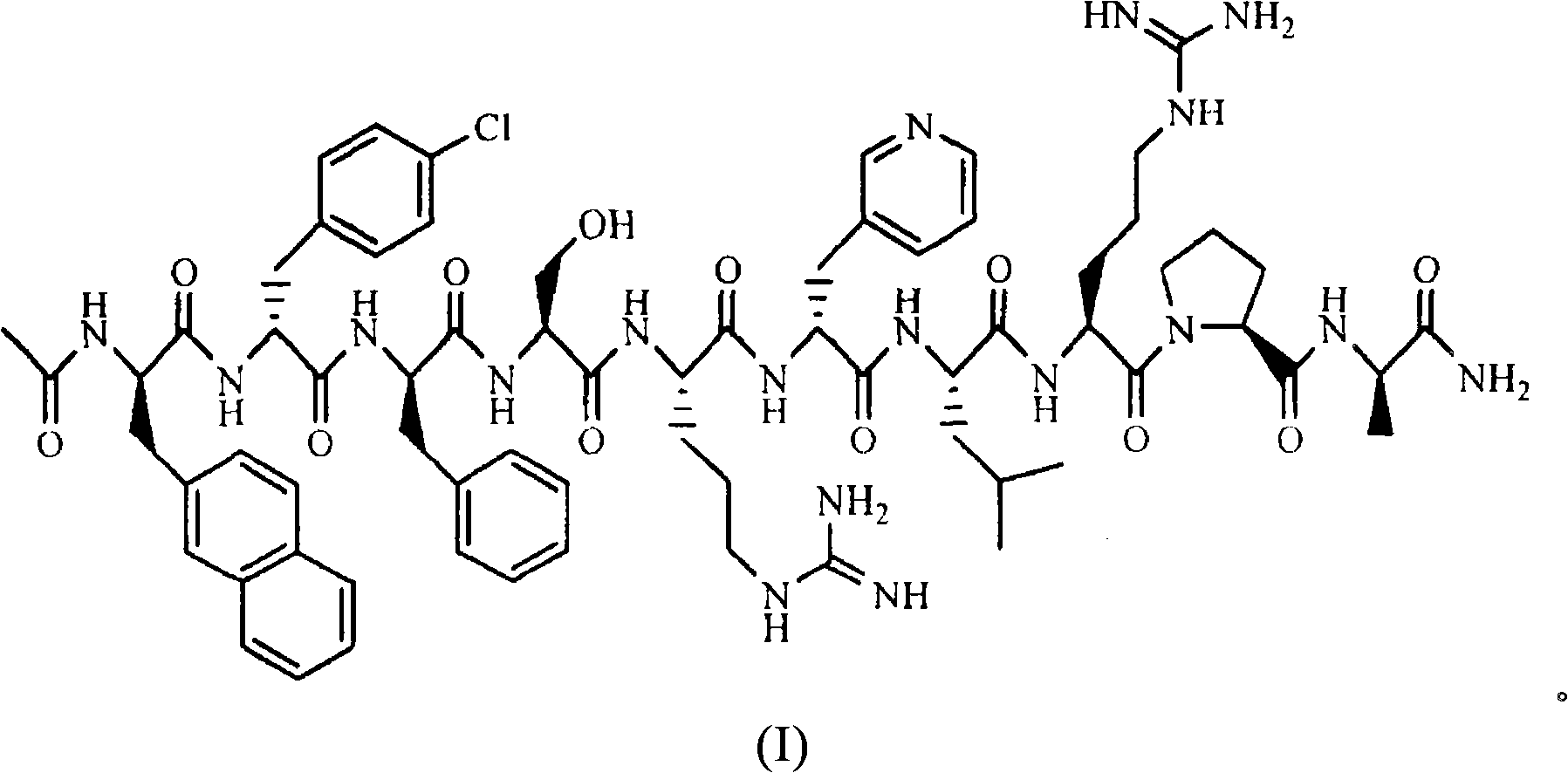
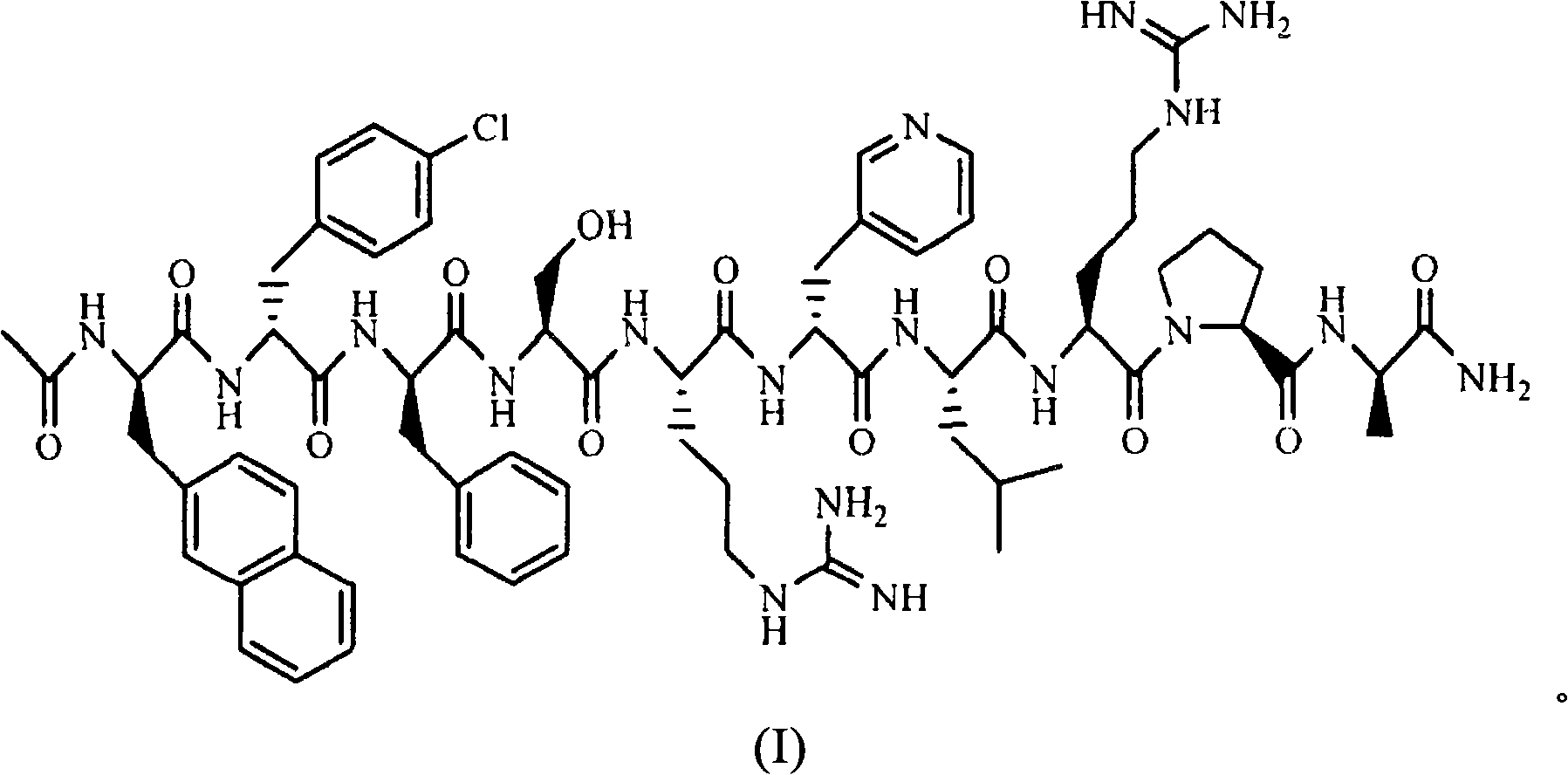
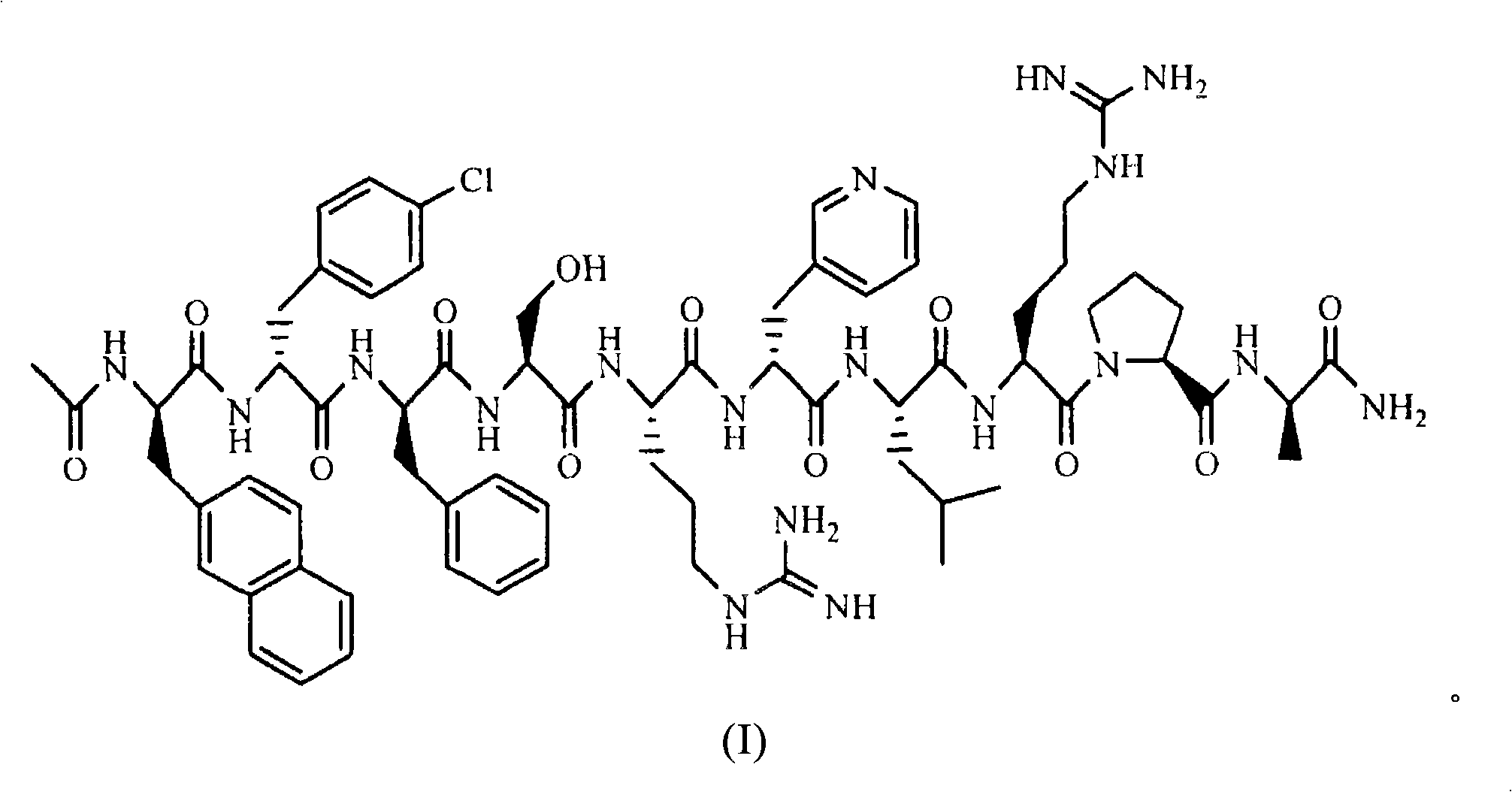
Sustained release preparation for injection of LHRH antagonist substances and preparation thereof
A technology for sustained-release preparations and injections, applied in the field of sustained-release preparations for injection of LXT101 and its preparation, which can solve the problems of short half-life, long course of treatment, pain of patients and inconvenience of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Preparation of formulation
[0048] The preparation of the present invention is prepared according to the following general method: Grind or pulverize LXT101 and pass it through a 100-mesh sieve, add it to the mixture of other ingredients, stir or grind, and use a high-pressure homogenizer to disperse, so that the particle size in the LXT101 suspension is different. If it exceeds 15μm, the preparation can be obtained by dispensing.
[0049] Different formulations as shown in Table 1 were prepared.
[0050] Table 1. Different formulations prepared by the present invention
[0051]
Embodiment 2
[0052] Example 2: Determination of cumulative release in vitro
[0053] Take the active ingredient reference substance and add water to prepare standard active ingredient solutions with concentrations of 10μg / mL, 20μg / mL, 30μg / mL, 50μg / mL, 100μg / mL, 200μg / mL, and measure the absorbance A by the Folin method. Absorbance A is used to regress the concentration, and a standard curve regression equation is established.
[0054] Take an appropriate amount of the prepared LXT101 sustained-release preparation and place it in a 50 mL conical flask with a stopper, and add 10 mL of a pH 7.2 PBS buffer solution to it. Place the Erlenmeyer flask in a constant temperature shaker at 37±1°C and oscillate at a frequency of 70r / m. At different time points, 200 μL of the liquid was quantitatively taken, and 200 μL of pH 7.2 PBS buffer solution was added at the same time. Centrifuge at 12,000r / m for 10 minutes, and take the supernatant as a sample solution. Measure the absorbance of the sample solu...
Embodiment 3
[0059] Example 3: Results of pharmacodynamic research in rats
[0060] Rats were given intramuscular injection (im) of the different LXT-101 preparations (including different doses) prepared in Example 1 according to Table 3 below, and the testosterone content (ng / ml) in the serum was determined at different times (days) after administration to Indirectly measure the release effect of LXT101 oily sustained-release preparation in vivo. The entire determination process is automatically completed by the BECKMAN COULTER Access Immunoassay System chemiluminescence instrument according to a predetermined program. The results are shown in Table 3 below.
[0061] The sustained release effect of the LXT-101 preparation was evaluated by measuring the testosterone level in the rat serum. From the results in Table 3, it can be seen that the preparation 1 can significantly inhibit the serum testosterone level for 5 to 7 days when the dose is 3.5 mg / kg; 2 When the dose is 3.5 mg / kg, it can sig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com