Method for preparing Ni base catalyst for methane portion oxidation synthesis gas
A catalyst and synthesis gas technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. Distributor loss and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
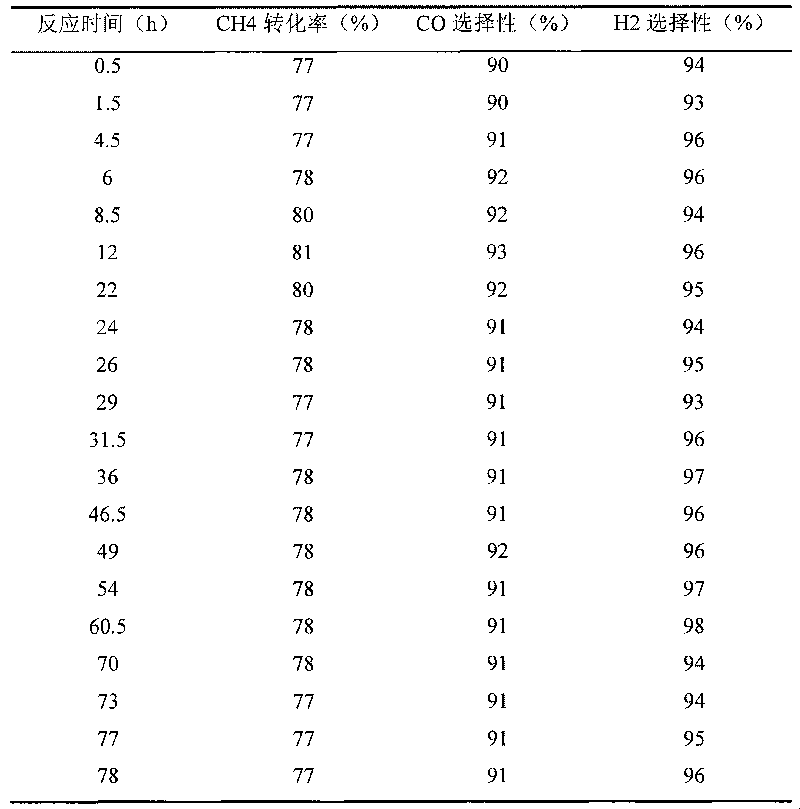
[0024] Ni / ZrO prepared by the above 1-2 steps 2 -0.1 g of HT-850-650 catalyst, carry out the partial oxidation reaction of methane according to the method described in step 3 above. V(CH 4 ) / V(O 2 ) = 2 reaction feed gas through the Ni / ZrO that has been reduced at 600°C at a flow rate of 165mL / min2 -HT-850-650 catalyst bed. The reaction tail gas flowing out from the reaction tube is analyzed by sampling at intervals of a certain period of time through the six-way valve, and the reaction is stopped after 78 hours of feeding the raw material gas. The result of embodiment 1 (the conversion rate of methane and product CO, H 2 selectivity) as shown in Table 1. Ni / ZrO after 78 hours of reaction 2 -The amount of carbon deposited on the HT-850-650 catalyst was analyzed by thermogravimetric (TG) method, and the results are listed in Table 7.
[0025] Table 1Ni / ZrO 2 -Results of partial oxidation of methane over HT-850-650 catalyst (reduction treatment at 600°C)
[0026]
Embodiment 2
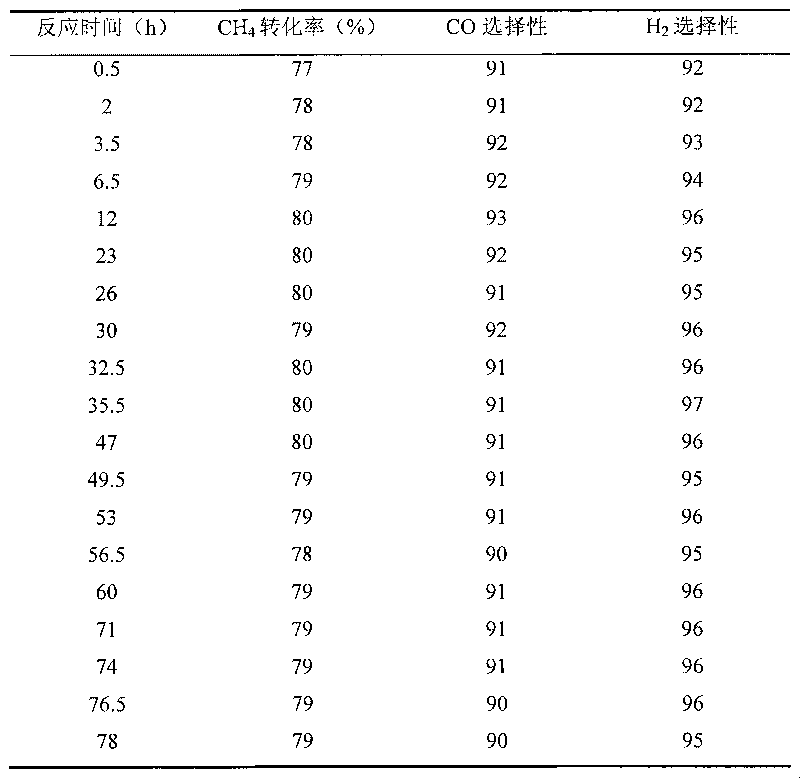
[0028] Except using Ni / ZrO 2 -HT-850-850 catalyst replaces Ni / ZrO in embodiment 1 2 -except HT-850-650 catalyzer, other steps are with embodiment 1. The reaction results are shown in Table 2. Ni / ZrO after 78 hours of reaction 2 -The amount of carbon deposited on the HT-850-850 catalyst was analyzed by thermogravimetric (TG) method, and the results are listed in Table 7.
[0029] Table 2Ni / ZrO 2 -Results of partial oxidation of methane over HT-850-850 catalyst (reduction treatment at 600°C)
[0030]
Embodiment 3
[0032] Catalyst removal (Ni / ZrO 2 -HT-850-850) adopts the "600°C+900°C reduction" in the above step 3, and other steps are the same as in Example 2. The reaction results are shown in Table 3. Ni / ZrO after 78 hours of reaction 2 -The amount of carbon deposited on the HT-850-850 catalyst was analyzed by thermogravimetric (TG) method, and the results are listed in Table 7.
[0033] Table 3Ni / ZrO 2 -Results of partial oxidation of methane over HT-850-850 catalyst (reduction treatment at 600°C+900°C)
[0034]
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com