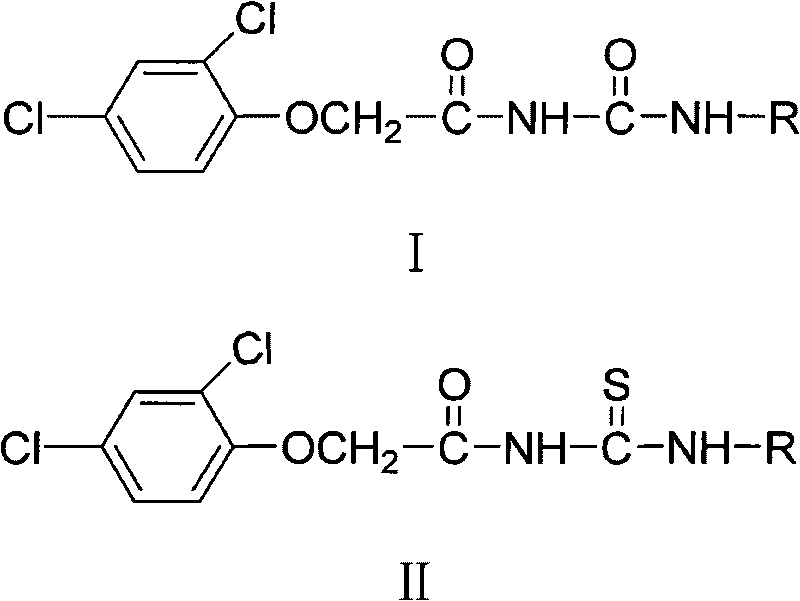
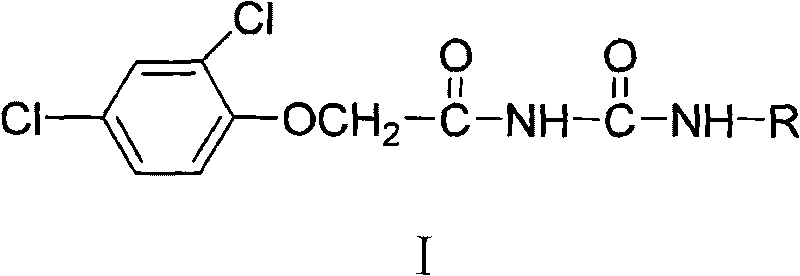
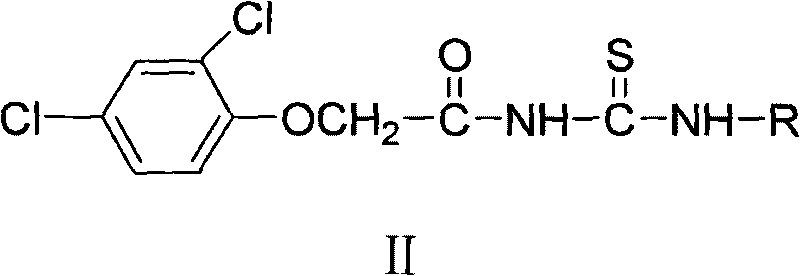
N-2, 4-dichlorophenoxy acetyl (sulfur) carbamide weedicide and preparation method thereof
A technology of dichlorophenoxyacetylthiourea and dichlorophenoxyacetylurea, which is applied in the N-2 field, can solve the problems of drug drift, influence on herbicidal effect, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Preparation of N-(2-tetrahydrofurylmethyl)-2,4-dichlorophenoxyacetylurea (I a ):
[0072] In a 50 mL round bottom flask, dissolve 2.21 g (0.01 mol) of 2,4-dichlorophenoxyacetic acid in 15 mL of freshly distilled toluene, add 2.9 mL (0.025 mol) of thionyl chloride dropwise, heat and reflux and stir for 2 hours, Toluene and excess thionyl chloride were removed under reduced pressure to obtain a yellow oil. The reaction solution was dropped into excess concentrated ammonia water, and white crystals were precipitated after thorough stirring. After washing with water and drying, 2,4-dichlorophenoxyacetamide was obtained.
[0073] Weigh 1.10g (0.005mol) of 2,4-dichlorophenoxyacetamide in a 50mL round-bottom flask and dissolve it in 15mL of toluene, weigh 2.62mL (0.03mol) of oxalyl chloride in an atmospheric dropping funnel and add it dropwise slowly , After reflux for 2 hours, toluene and excess oxalyl chloride were removed under reduced pressure to obtain a crude product ...
Embodiment 2
[0079] Preparation of N-(2-furylmethyl)-2,4-dichlorophenoxyacetylurea (I b ):
[0080] In a 50 mL round bottom flask, dissolve 2.21 g (0.01 mol) of 2,4-dichlorophenoxyacetic acid in 15 mL of freshly distilled toluene, add 2.9 mL (0.025 mol) of thionyl chloride dropwise, heat and reflux and stir for 2 hours, Toluene and excess thionyl chloride were removed under reduced pressure to obtain a yellow oil. The reaction solution was dropped into excess concentrated ammonia water, and white crystals were precipitated after thorough stirring. After washing with water and drying, 2,4-dichlorophenoxyacetamide was obtained.
[0081] Weigh 1.10g (0.005mol) of 2,4-dichlorophenoxyacetamide in a 50mL round-bottom flask and dissolve it in 15mL of toluene, weigh 2.62mL (0.03mol) of oxalyl chloride in an atmospheric dropping funnel and add it dropwise slowly , After reflux for 2 hours, toluene and excess oxalyl chloride were removed under reduced pressure to obtain a crude product of isocyan...
Embodiment 3
[0087] Preparation of N-(2-pyridylmethyl)-2,4-dichlorophenoxyacetylurea (I c ):
[0088] In a 50 mL round bottom flask, dissolve 2.21 g (0.01 mol) of 2,4-dichlorophenoxyacetic acid in 15 mL of freshly distilled toluene, add 2.9 mL (0.025 mol) of thionyl chloride dropwise, heat and reflux and stir for 2 hours, Toluene and excess thionyl chloride were removed under reduced pressure to obtain a yellow oil. The reaction solution was dropped into excess concentrated ammonia water, and white crystals were precipitated after thorough stirring. After washing with water and drying, 2,4-dichlorophenoxyacetamide was obtained.
[0089] Weigh 1.10g (0.005mol) of 2,4-dichlorophenoxyacetamide in a 50mL round-bottom flask and dissolve it in 15mL of toluene, weigh 2.62mL (0.03mol) of oxalyl chloride in an atmospheric dropping funnel and add it dropwise slowly , After reflux for 2 hours, toluene and excess oxalyl chloride were removed under reduced pressure to obtain a crude product of isocy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com