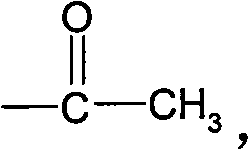
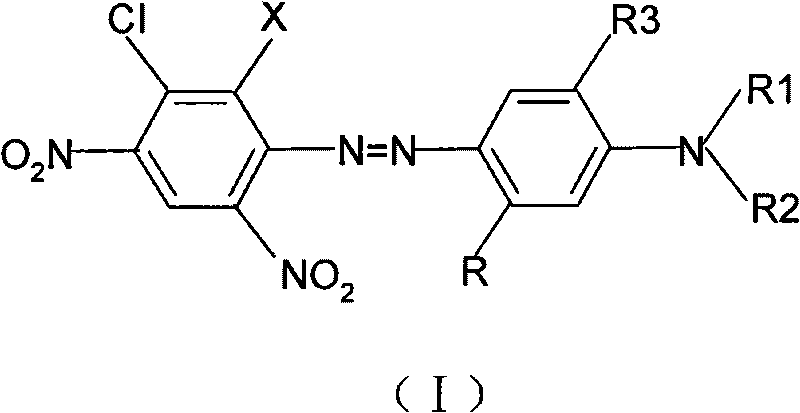
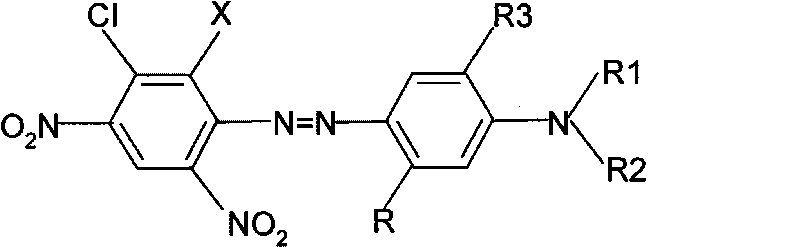
5-chloronitroaniline-containing azoic disperse dyes and preparation method and application thereof
A chloronitroaniline, disperse dye technology, applied in azo dyes, monoazo dyes, dyeing methods and other directions, can solve problems such as harm to health, unstable dye crystal form, environmental pollution, etc., to achieve environmental friendly, wet Excellent handling fastness and sublimation fastness, the effect of stable dye crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] Preparation of diazo compounds:
[0035] (1) Add 250ml of concentrated sulfuric acid to a 1000ml flask, add 65ml of fuming nitric acid at room temperature, exotherm, the temperature rises to 40°C, add 70ml of m-dichlorobenzene dropwise, control the feeding speed and cool down with a water bath, so that the feeding temperature is controlled at 60- 75°C, 1-1.5 hours to complete the addition. Raise the temperature to 80-85°C, and keep it warm for 3 hours. Then add 125ml of 50% fuming sulfuric acid dropwise at a temperature of 80-85°C and finish adding in 1.5 hours. After maintaining the reaction at this temperature for 3 hours, the temperature was then raised to 100-110° C., and the reaction was continued for 3 hours. When the mononitride content is lower than 1.0%, the reaction is terminated, and the temperature is cooled to room temperature. Add water and a large amount of ice to a 2000ml beaker, control the temperature below 10°C, slowly add the above reaction soluti...
Embodiment 1
[0040] Add 30ml of concentrated sulfuric acid and 34g of 40% nitrosylsulfuric acid into a 250ml flask, stir, cool down to 10-15°C in an ice-water bath, slowly add 21.8g of 2,4-dinitro-5-chloroaniline, and control the temperature for 10 -15°C, 20-30min to complete the addition. And keep it warm at this temperature for 3 hours and set it aside. Add 200ml of water, 9ml of concentrated sulfuric acid, and 1g of sulfamic acid into a 2000ml beaker, stir evenly, add 0.102mol of dark blue esterification solution and crushed ice, add the above diazo solution dropwise in the range of 0-5°C, and add sodium acetate to adjust pH=2.0-2.5, stirred for 1 hour, the diazo component disappeared, raised the temperature to 35-40°C, kept it warm for 1 hour, filtered and washed with water until neutral. The blue dye compound represented by formula (I-1) was obtained.
[0041]
[0042] (I-1)
Embodiment 2
[0044] At room temperature, put 90ml of water, 36g of 4-methoxy-3-aminoacetanilide, 122g of β-methoxyethyl p-toluenesulfonate, and 12g of magnesium oxide into a 250ml flask in sequence, start stirring, and slowly Raise the temperature to 80-85°C, react for 6 hours, then raise the temperature to 92-95°C and keep the reaction for 12 hours. When the content of the main product is ≥ 96% as detected by HPLC, cool down to 30°C, filter, and wash with water to obtain 4-methoxy-3 -N,N-bis(β-methoxyethyl)aminoacetanilide.
[0045]According to the preparation method described in Example 1, 0.1mol of 2,4-dinitro-5-chloroaniline and 4-methoxy-3-N, N-di(β-methoxyethyl)aminoacetanilide 0.102mol reaction can prepare the dye compound shown in formula (I-2).
[0046]
[0047] (I-2)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com