Process for obtaining steroidal phosphate compounds
A technology of compounds and phosphate esters, used in pharmaceutical formulations, drug combinations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
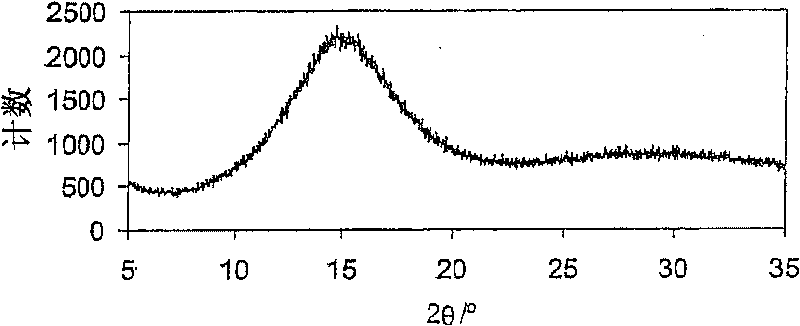
[0067] Embodiment 1: the spray drying of betamethasone 21-phosphate disodium
[0068] Wet betamethasone 21-phosphate disodium having a purity of 99.8% (HPLC area %) obtained by applying literature techniques was dissolved in water to obtain a 5% by weight solution on dry matter basis. The pH of the solution was adjusted to 7.6 / 7.9 by adding 1N hydrochloric acid. The outlet temperature was maintained between 80°C and 100°C, the atomization flow rate was 357-670 standard liters per hour, and the solution flow rate was between 5ml / min and 9ml / min. The product was collected in a high performance cyclone. The product [betamethasone 21-phosphate] was obtained with a purity of 99.6% and residual solvents meeting the requirements of the USP and EP pharmacopoeias (methanol: 428 ppm; isopropanol: 2088 ppm; water, by Karl-Fischer method: 8.0% by weight).
Embodiment 2
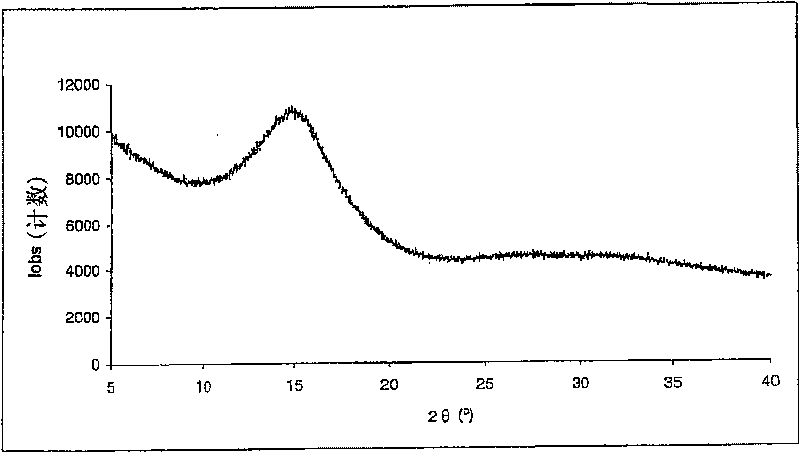
[0069] Embodiment 2: the spray drying of dexamethasone 21-phosphate disodium
[0070] Dexamethasone disodium 21-phosphate having a purity of 99.2% (HPLC area %) was dissolved in water to obtain a 5% by weight solution. The pH of the solution was adjusted to 7.6 / 7.9 by adding 1N hydrochloric acid. The outlet temperature was maintained between 80°C and 100°C, the atomization flow rate was 357-670 standard liters per hour, and the solution flow rate was between 5ml / min and 9ml / min. The product was collected in a high performance cyclone. The product [dexamethasone 21-phosphate] was obtained with a purity of 99.2% (HPLC area %).
Embodiment 3
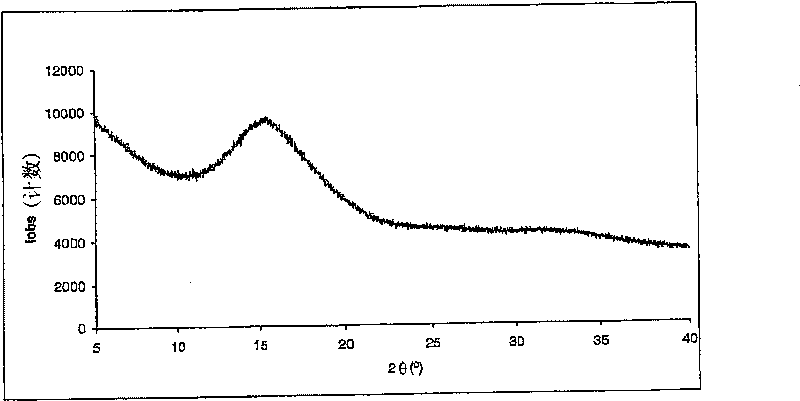
[0071] Embodiment 3: the spray drying of prednisolone 21-phosphate disodium
[0072] Disodium prednisolone 21-phosphate with a purity of 98.4% (HPLC area %) was dissolved in water to obtain a 5% by weight solution. The pH of the solution was adjusted to 7.6 / 7.9 by adding 1N hydrochloric acid. The outlet temperature was maintained between 80°C and 100°C, the atomization flow rate was 357-670 standard liters per hour, and the solution flow rate was between 5ml / min and 9ml / min. The product was collected in a high performance cyclone. The product [prednisolone 21-phosphate] was obtained with a purity of 99.2% (HPLC area %).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com