Isosorbide mononitrate controlled release tablet and preparation method thereof
A technology of isosorbide dinitrate and controlled-release tablets, which is applied in the field of medicine to achieve the effects of small fluctuations in blood drug concentration, stable drug release, and long-lasting drug effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
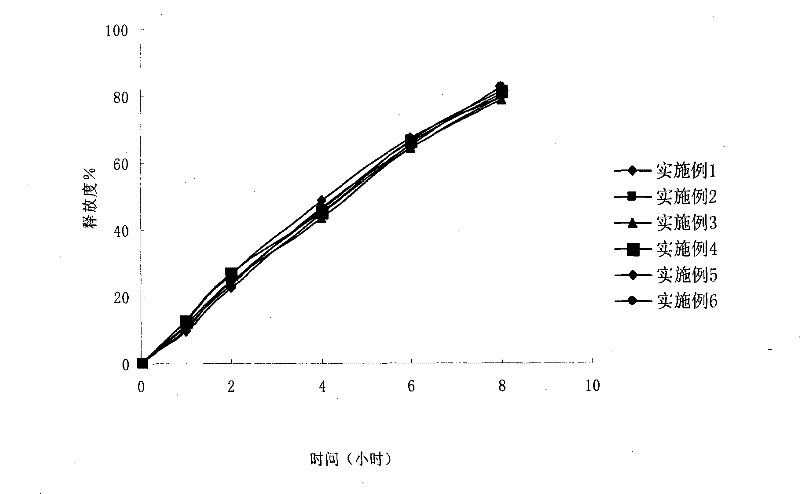
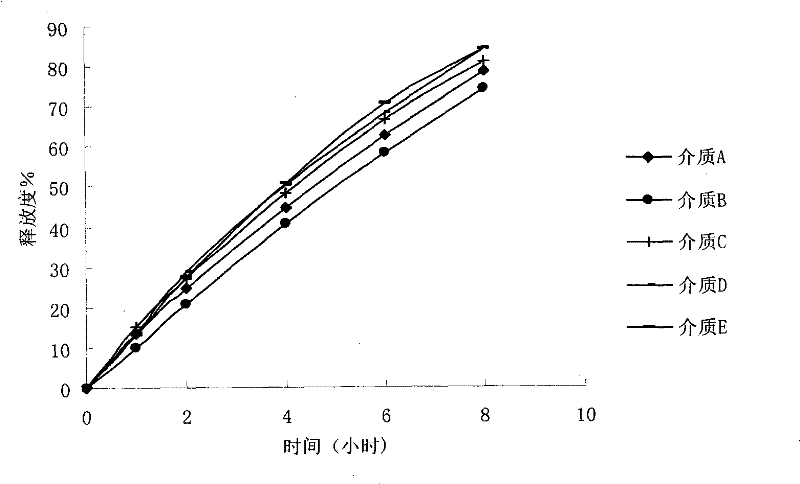
Examples
preparation example Construction
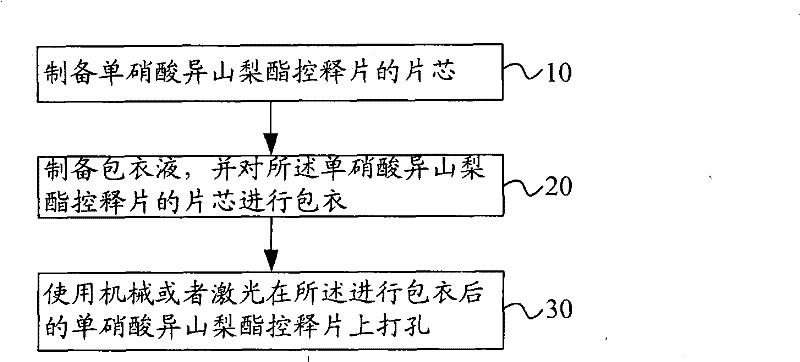
[0039] figure 1 It is the flow chart of the preparation method embodiment of isosorbide mononitrate controlled-release tablet of the present invention. Such as figure 1 As shown, the method includes the following steps:
[0040] Step 10: preparing the core of the isosorbide mononitrate controlled-release tablet.
[0041] Described step 10 comprises the following steps:
[0042] Step 101: Pass isosorbide mononitrate and the first auxiliary material through a 40-70 mesh sieve respectively, and mix evenly;
[0043] Step 102: After passing the second auxiliary material through a 10-20 mesh sieve, add it to the uniformly mixed isosorbide mononitrate and the first auxiliary material, and mix evenly;
[0044] Step 103: Compress the uniformly mixed isosorbide mononitrate, first auxiliary material and second auxiliary material to form a tablet core.
[0045] Step 20: Prepare a coating solution, and coat the core of the isosorbide mononitrate controlled-release tablet.
[0046] Af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com