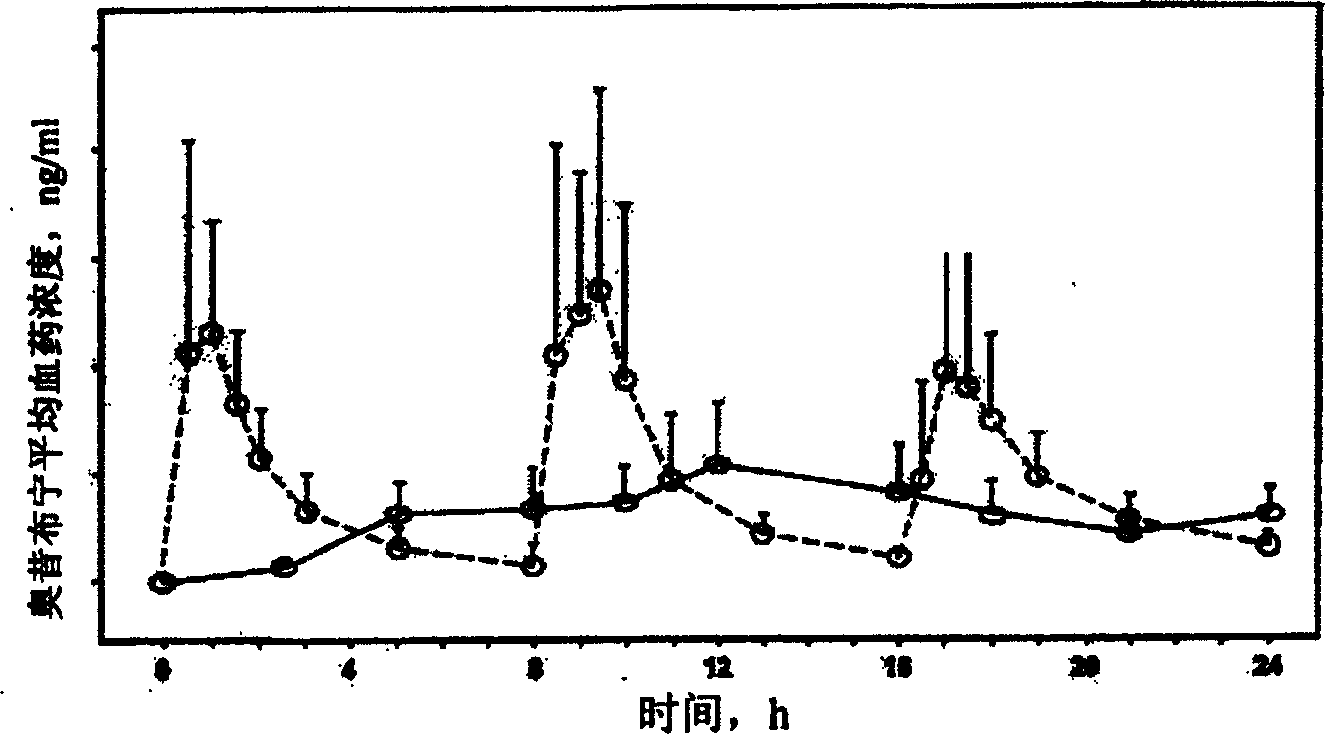
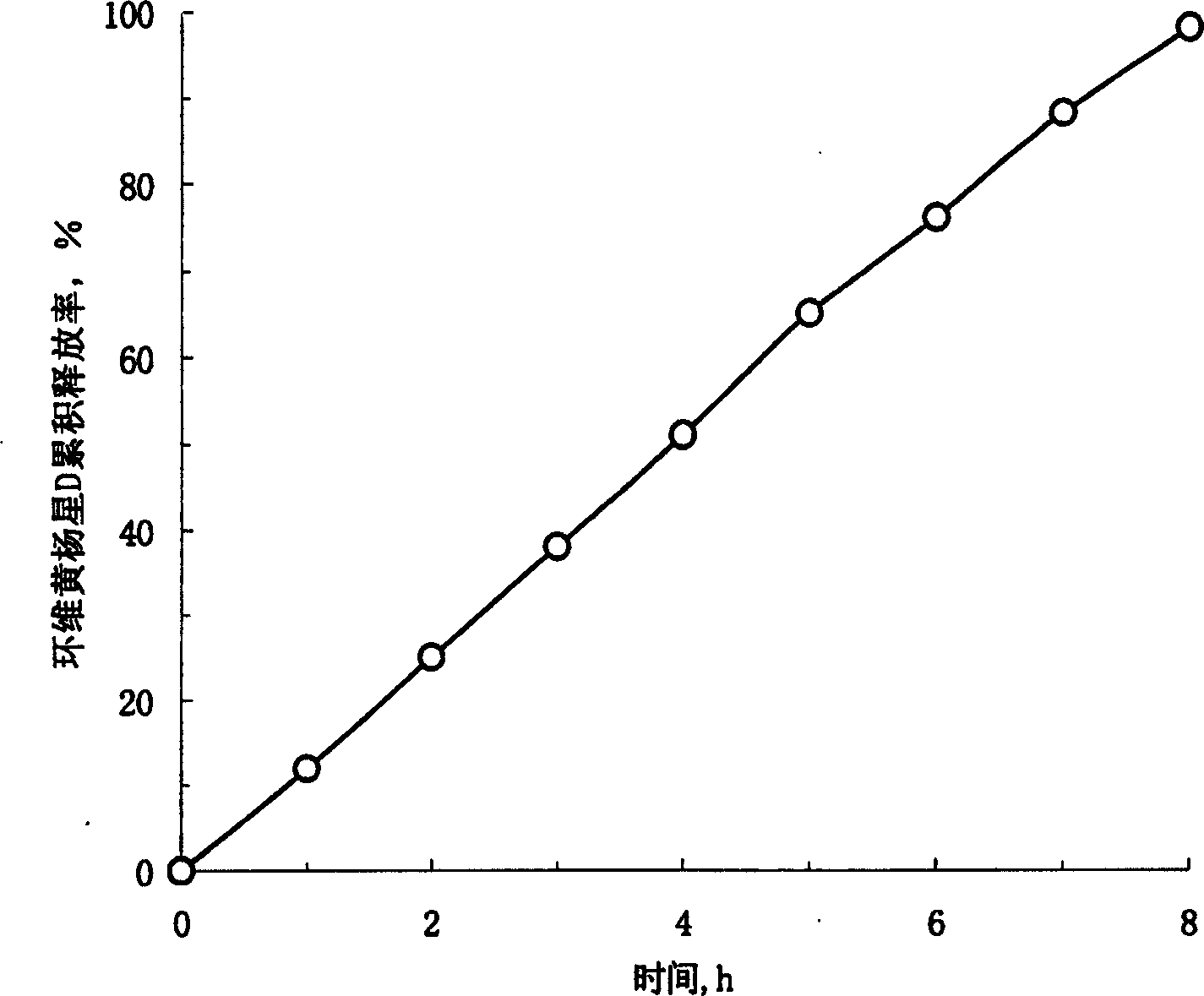
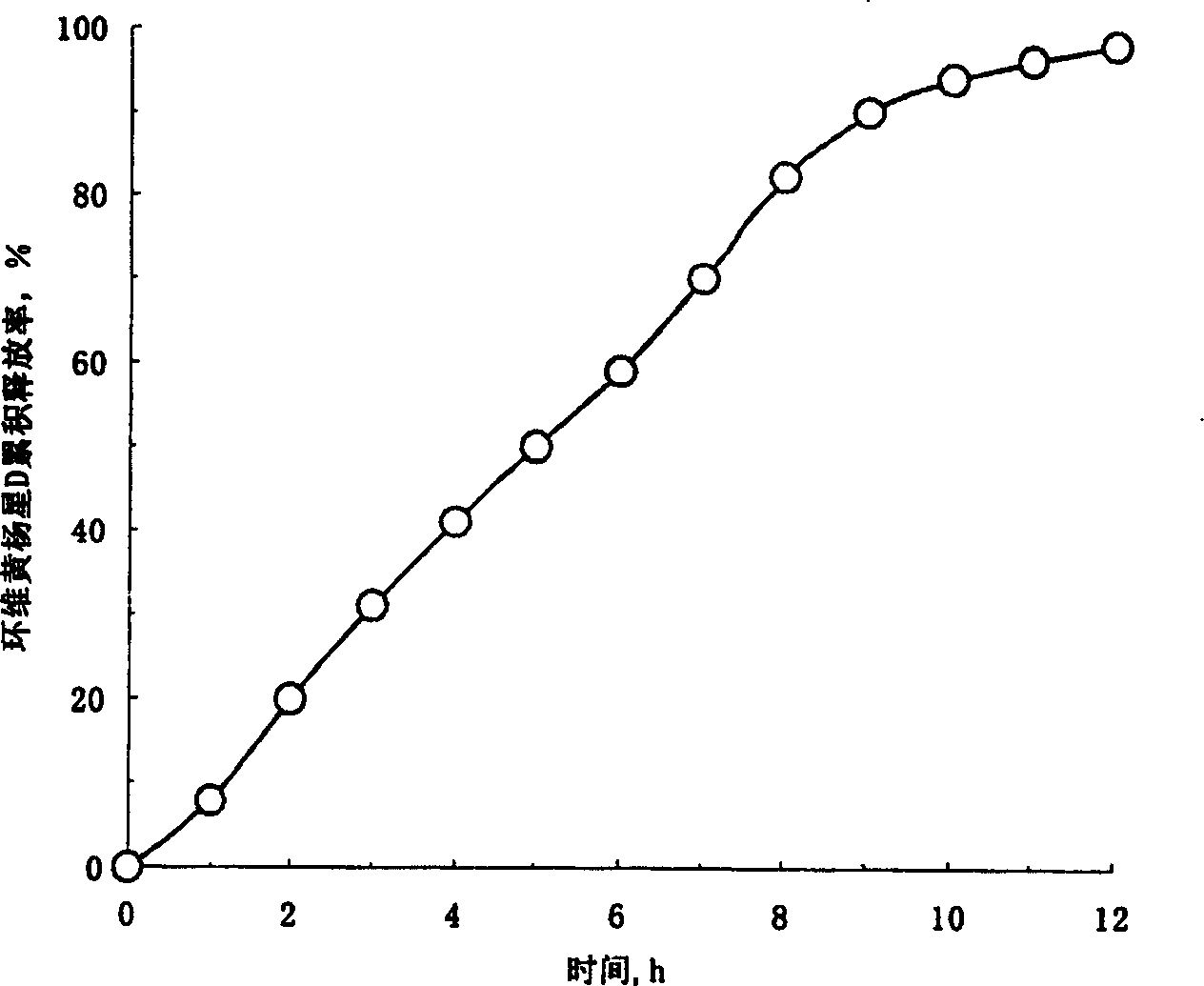
Osmosis pump controlled release preparation containing Chinese medicine of cyclovirobuxine D, and preparing method thereof
A technology of cyclovir buxus and osmotic pump controlled release, which is applied in the field of traditional Chinese medicine formulations and can solve the problems of high equipment requirements, complicated preparation process of dual-chamber osmotic pump tablets, and difficulty in popularizing industrialized production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0053] Example 1: Weigh 3.0g of Cycloviral Buxus D, 25.0g of sodium chloride, 15.0g of sucrose, 12.0g of lactose, 23.0g of glucose, 26.0g of mannitol, 5.2g of succinic acid, 4.3g of sodium carboxymethyl starch, paste Refined 12.0g, polyethylene oxide 1.5g.
[0054] The above components are passed through 80 mesh sieve respectively, and mixed evenly. Granulate with 20% polyvinylpyrrolidone ethanol solution, pass through a 20-mesh sieve and dry in an oven at 60° C. for 18 hours. After taking out, granulate with 20 mesh sieves, add 1% magnesium stearate as a lubricant, and press it into a tablet core with a hardness of 90-100N.
[0055] Weigh 5.5g of carboxypropylmethylcellulose, 4.8g of cellulose acetate, and 0.5g of polyethylene glycol, and dissolve them in 200ml of acetone. The obtained coating solution is evenly applied on the tablet core, so that the weight gain is 6.5 mg / tablet to 8.0 mg / tablet. A small hole with a diameter of 0.4 mm to 0.8 mm is punched on both sides of...
example 2
[0056] Example 2: Weigh 4.0g of Cycloviral Buxus D, 45.0g of sodium chloride, 30.0g of sucrose, 24.0g of lactose, 30.0g of glucose, 40.0g of mannitol, 5.6g of tartaric acid, 4.5g of carmellose, carboxymethylcellulose Sodium starch glycolate 6.0g, dextrin 20.0g, polyethylene oxide 2.5g.
[0057] The above components are passed through 80 mesh sieve respectively, and mixed evenly. Granulate with 20% polyvinylpyrrolidone ethanol solution, pass through a 20-mesh sieve and dry in an oven at 60° C. for 18 hours. After taking out, granulate with 20 mesh sieves, add 1% magnesium stearate as a lubricant, and press it into a tablet core with a hardness of 90-100N.
[0058] Weigh 6.5g of carboxypropylmethylcellulose, 5.8g of cellulose acetate, and 1.5g of polyethylene glycol, and dissolve them in 300ml of acetone. The obtained coating solution is evenly applied on the tablet core, so that the weight gain is 6.5 mg / tablet to 7.5 mg / tablet. A small hole with a diameter of 0.4 mm to 0.8 ...
example 3
[0059] Example 3: Weigh 6.0g of Cycloviral Buxusin D, 65.0g of sodium chloride, 40.0g of sucrose, 36.0g of lactose, 35.0g of glucose, 60.0g of mannitol, 9.6g of sodium dihydrogen phosphate, and 5.5g of carmellose , sodium carboxymethyl starch 8.0g, dextrin 32.0g, polyethylene oxide 4.5g.
[0060] The above components are passed through 80 mesh sieve respectively, and mixed evenly. Granulate with 20% polyvinylpyrrolidone ethanol solution, pass through a 20-mesh sieve and dry in an oven at 60° C. for 18 hours. After taking out, granulate with 20 mesh sieves, add 1% magnesium stearate as a lubricant, and press it into a tablet core with a hardness of 90-100N.
[0061] Weigh 6.5g of carboxypropylmethylcellulose, 7.8g of cellulose acetate, and 1.75g of polyethylene glycol, and dissolve them in 300ml of acetone. The obtained coating solution is uniformly applied on the tablet core, so that the weight gain is 5.5 mg / tablet to 6.5 mg / tablet. A small hole with a diameter of 0.4 mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com