Compound containing acetyl pyrethroid, synthesis method and application thereof
A technology for pyrethroids and synthetic methods, which is applied in the field of organic insecticidal pyrethroids, can solve problems such as insufficient insecticidal effects, and achieve the effects of easy control of the process, less stimulation to humans and animals, and good drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
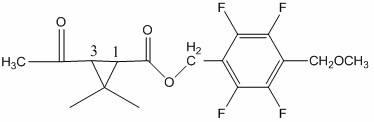
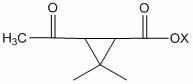
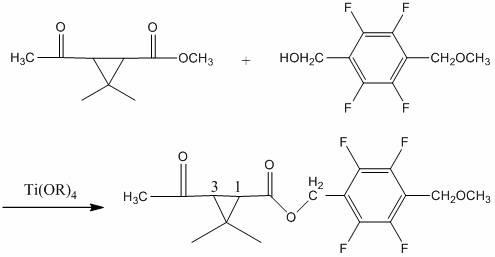
[0068] Add 18.0 g of methyl 3-acetyl-2,2-dimethylcyclopropanecarboxylate and 2,3,5,6-tetrafluoro -22.4 g of 4-methoxymethylbenzyl alcohol, add Ti(OC 2 h 5 ) 4 3 g, 100 g of toluene, heated up to 110 °C, kept the temperature for 4 hours, the reaction was completed, lowered to room temperature, added water to wash once, added toluene for extraction, combined oil layer precipitation, recovered toluene, and obtained 34.2 g of light yellow ester, the content 96.7%, yield 91.36%.
Embodiment 2
[0070] Add 20.5 g of ethyl 3-acetyl-2,2-dimethylcyclopropanecarboxylate and 2,3,5,6-tetrafluoro -22.4 g of 4-methoxymethyl benzyl alcohol, add Ti(OC 3 h 7 ) 4 6 g, 100 g heptane, heat up to 120 °C, keep warm for 5 hours, after the reaction is completed, lower to room temperature, add water to wash once, add heptane to extract once, combine oil layers for precipitation, recover heptane, and obtain light yellow ester 34.4 g, content 95.7%, yield 90.94%.
Embodiment 3
[0072] Add 22.3 g of 3-acetyl-2,2-dimethylcyclopropanecarboxylic acid propyl ester and 2,3,5,6-tetra Fluoro-4-methoxymethyl benzyl alcohol 22.4 g, add Ti(OCH 3 ) 4 4 g, 100 g cyclohexane, heat up to 80 ° C, keep warm for 10 hours, after the reaction is completed, lower to normal temperature, add water to wash once, add cyclohexane to extract once, combine oil layers for precipitation, recover cyclohexane, and obtain Yellow ester 35.0 g, content 95.1%, yield 91.94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com