Actinopolyspora erythraea
A technology of actinomycetes and erythromycin, which is applied in the field of microbiology and can solve the problems of reduced curative effect of erythromycin drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
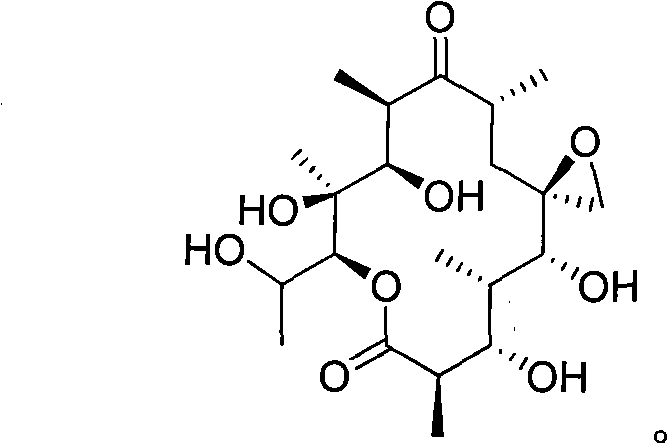
[0022] Use the erythromycin-producing polyspore actinomycete Actinopolyspora erythraea YIM 90600 of the present invention T Preparation of new compound 6,18-epoxy-14hydroxyerythromycin lactone B.
[0023] 1. Actinopolyspora erythraea YIM 90600 T Fermentation treatment of strains:
[0024] a. Cultivate ISP 4+10% NaCl on a slant, and cultivate at 37°C for 15 days until the growth of aerial spores;
[0025] b. Seed culture ISP 4 (no agar) + 10% NaCl, 37 ° C, 250 rpm, culture for 10 days;
[0026] c. Fermentation culture In the fermentation medium, at 37 degrees Celsius, 250 rpm, cultivated for 30 days, the composition and proportion of the fermentation medium: soybean powder 20g, peptone 2g, glucose 20g, starch 5g, yeast extract 2g, NaCl 74g , K 2 HPO 4 0.5g, MgSO 4 ·7H 2 O 0.5g, Na 2 SO 4 10H 2 O 25g, KCl 15g, MgCl 2 ·6H 2 O 10g, CaSO 4 2H 2 O 5g, CaCO 3 2g, 1000ml deionized water, pH 7.8;
[0027] Operation process: Take a spore of the strain that has grown in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com