Biological dehydrogenation preparation method of 6 alpha-methylprednisolone intermediate
A kind of intermediate and biological technology, which is applied in the field of preparation of steroidal drug intermediates, can solve the problems of differences in the production of dehydrogenates, no disclosure of strain preservation information, technical solutions that cannot be implemented, etc., to achieve the effect of avoiding residues and flexible selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
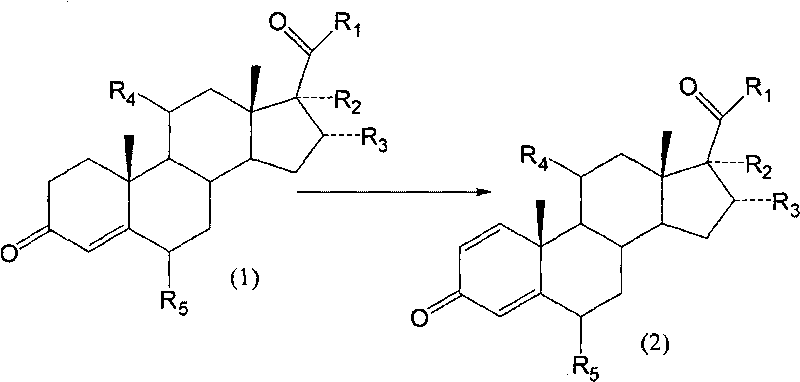
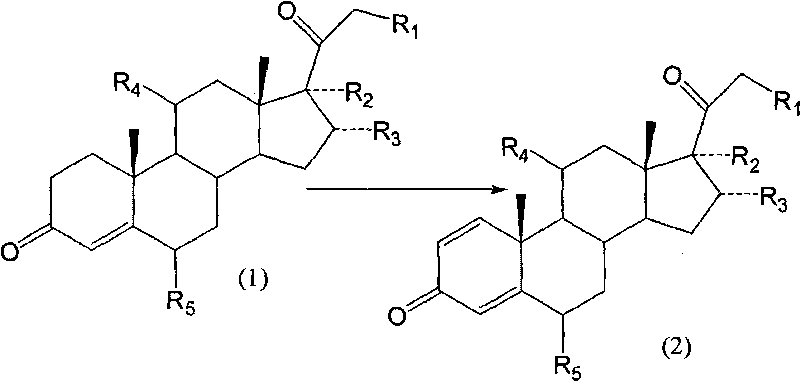
Image
Examples
Embodiment 1
[0049] Example 1: Using 11β, 17α-dihydroxy-pregna-4-ene-3,20-dione as a substrate;
[0050] Arthrobacter simplex (AS 1.94*) was subjected to slant culture, primary culture and secondary culture in sequence. The culture temperature was 31°C. The substrate dissolved in DMF was put into a 5L fermenter, the feeding concentration was 1%, and the reaction temperature was 31°C. The reaction time was 60 hours. After the reaction was completed, the temperature was raised to 70° C. to terminate the reaction. The fermentation broth was extracted with ethyl acetate, and the organic phase was concentrated. The conversion rate of the substrate was measured to be 73.5%.
Embodiment 2
[0051]Example 2: Using 17α, 21-dihydroxy-pregn-4-ene 3,11,20-triketone-21-acetate as the substrate;
[0052] Arthrobacter simplex (AS 1.94*) was subjected to slant culture, primary culture and secondary culture in sequence. The culture temperature was 32°C. The substrate dissolved in tetrahydrofuran was put into a 5L fermenter, the feeding concentration was 2%, and the reaction temperature was 32°C. The reaction time was 48 hours. After the reaction was completed, the temperature was raised to 70° C. to terminate the reaction. The fermentation broth was extracted with ethyl acetate, and the organic phase was concentrated. The conversion rate of the substrate was measured to be 77.3%.
Embodiment 3
[0053] Example 3: Using 11β, 17α, 21-trihydroxy-pregna-4-ene-3,20-dione-21-acetate as the substrate;
[0054] Arthrobacter simplex (AS 1.754) was subjected to slant culture, primary culture and secondary culture in sequence, and the culture temperature was 33°C. The substrate dissolved in dioxane was put into a 5L fermenter, and the feeding concentration was 1%, and the reaction The temperature was 33°C. The reaction time was 36 hours. After the reaction was completed, the temperature was raised to 70° C. to terminate the reaction. The fermentation broth was extracted with ethyl acetate, and the organic phase was concentrated. The conversion rate of the substrate was measured to be 81.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com