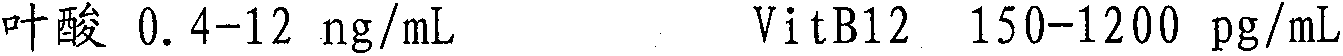Multinomial liquid quality control material and preparation method thereof
A technology of quality control substances and liquids, applied in the field of medical inspection quality control, can solve the problems of pathogenic microorganisms, difficulty in meeting actual needs, and few quality control components, and achieve a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Step 1: Collect blood waste from clinical tests, which requires normal liver function, negative HBsAg, no hemolysis and jaundice;
[0052] Step two, separate the serum from the blood;
[0053] Step 3: Analyze and determine the content of each quality control component in the serum according to conventional methods;
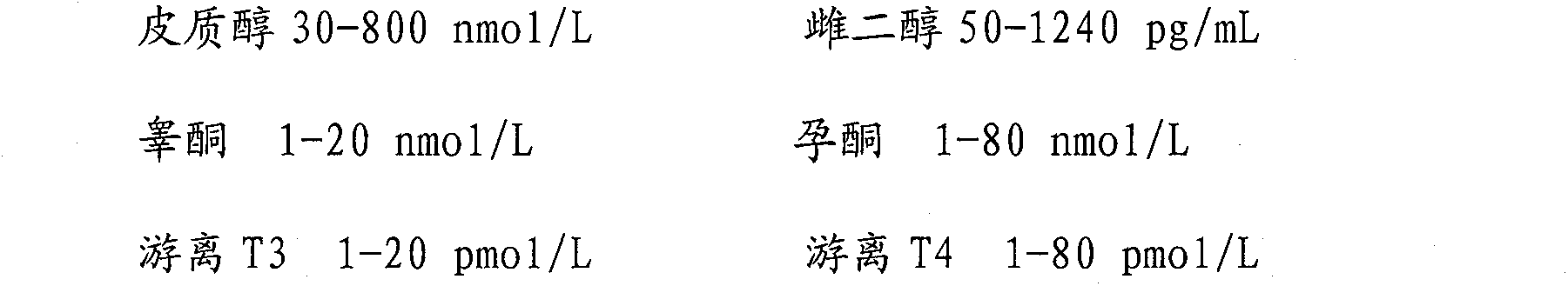
[0054] Step 4: Adjust the content of each analytical component according to the requirements of the quality control substance, and add the corresponding substances to achieve the required concentration;
[0055] Step 5, inactivate various potential pathogenic microorganisms by using the methylene blue photosensitive inactivation method;
[0056] Step 6, adding a composite protective agent composed of ethylene glycol and glycerol with a volume ratio of 1:1 accounting for 30% of the total volume of the quality control substance to prepare a quality control substance, wherein the ethylene glycol and glycerol are analytically pure;
[0057] Step 7, analyze the content of ...
Embodiment 2
[0061] Step 1: Collect fresh pig blood;
[0062] Step two, separate the serum from the blood;
[0063] Step 3: Analyze and determine the content of each quality control component in the serum according to conventional methods;
[0064] Step 4: Adjust the content of each analytical component according to the requirements of the quality control substance, and add the corresponding substances to achieve the required concentration;
[0065] Step 5, inactivate various potential pathogenic microorganisms by using the methylene blue photosensitive inactivation method;
[0066] Step 6, adding a composite protective agent composed of ethylene glycol and glycerol with a volume ratio of 1:1 accounting for 40% of the total volume of the quality control substance to prepare a quality control substance, wherein the ethylene glycol and glycerol are analytically pure;
[0067] Step 7, analyze the content of each quality control component in the quality control and determine its value;
[0068] Step 8, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com