Injection packaging preparation of thymosin pha1 microspheres
A thymosin, subpackaging technology, applied in the direction of peptide/protein components, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to achieve the effect of ensuring accuracy and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
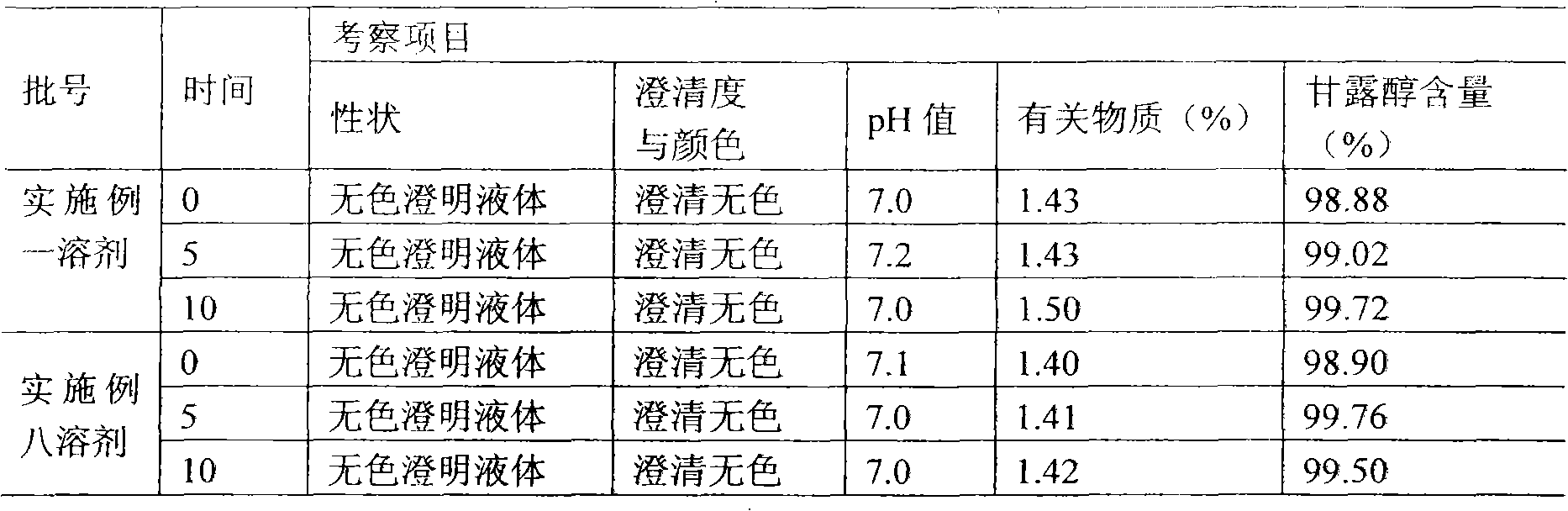
[0032] Example 1 Preparation of Thymosin α1 Microsphere Injection Packing Preparation
[0033] (1) Preparation of special injection solvent
[0034] (1) Prescription
[0035] CMC-Na (molecular weight 300-800) 5g
[0036] Mannitol 25g
[0037] Add water for injection to 1000ml
[0038] (2) Accurately weigh each auxiliary material according to the prescription quantity;
[0039] (3) Take the required mannitol in a suitable container between the ingredients under 100-level laminar flow, add about 900ml of sterilized water for injection to dissolve, stir, and mix evenly, and measure the required carboxymethyl alcohol according to the amount of feed Sodium cellulose, slowly add to the container while stirring, continue to stir until the solution is clear, add water for injection to 1000ml.
[0040] (4) Filter the prepared medicinal solution and wait for subpackaging.
[0041] (5) Before subpackaging, use sterile water for injection to adjust the dosage according to 5.0ml per vi...
Embodiment 2
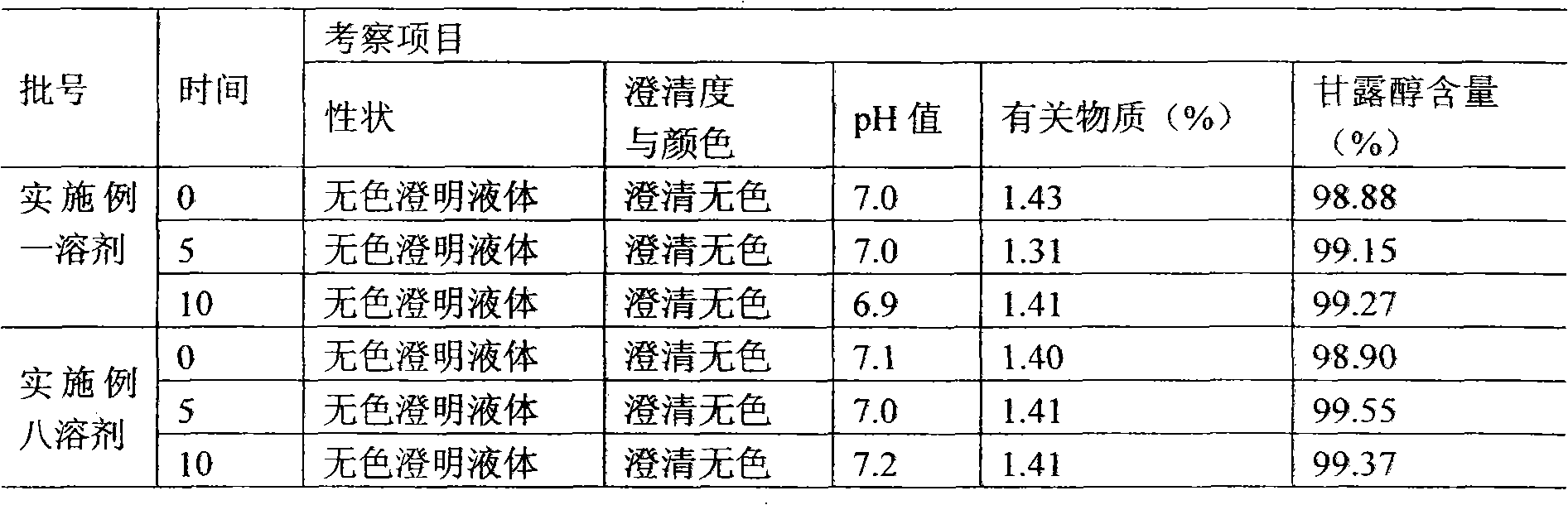
[0046] Example 2 Preparation of Thymosin α1 Microsphere Injection Packing Preparation
[0047] (1) Preparation of special injection solvent
[0048] (1) Prescription
[0049] CMC-Na (molecular weight 800-1200) 2.5g
[0050] Mannitol 25g
[0051] Add water for injection to 1000ml
[0052] (2) Accurately weigh each auxiliary material according to the prescription quantity;
[0053] (3) Take the required mannitol in a suitable container between the ingredients under 100-level laminar flow, add about 900ml of sterilized water for injection to dissolve, stir, and mix evenly, and measure the required carboxymethyl alcohol according to the amount of feed Sodium cellulose, slowly add to the container while stirring, continue to stir until the solution is clear, add water for injection to 1000ml.
[0054] (4) Filter the prepared medicinal solution and wait for subpackaging.
[0055] (5) Before dispensing, use sterile water for injection to adjust the dosage according to 3.0ml per...
Embodiment 3
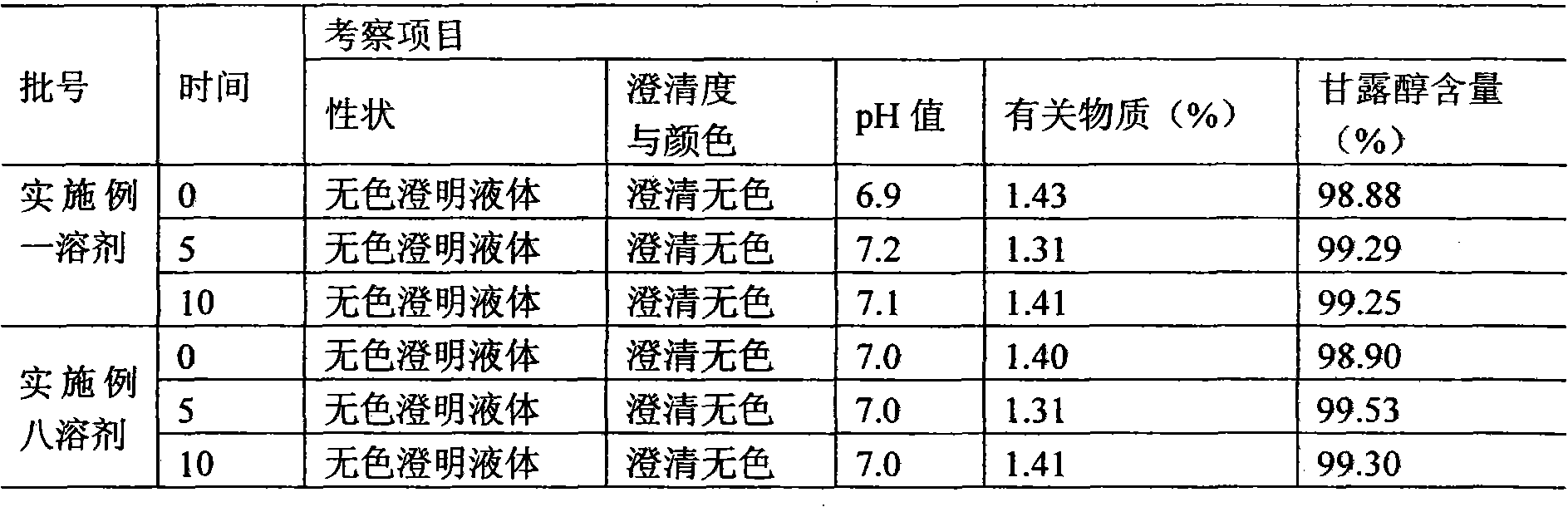
[0060] Example 3 Preparation of Thymosin α1 Microsphere Injection Packing Preparation
[0061] (1) Preparation of special injection solvent
[0062] (1) Prescription
[0063] Carbomer 2g
[0064] Mannitol 25g
[0065] Add water for injection to 1000ml
[0066] (2) Accurately weigh each auxiliary material according to the prescription quantity;
[0067] (3) Take the required mannitol in a suitable container between the ingredients under 100-level laminar flow, add about 900ml of sterilized water for injection to dissolve, stir, and mix evenly, and measure the required carbomer according to the amount of feed , slowly add to the container while stirring, continue to stir until the solution is clear, add water for injection to 1000ml.
[0068] (4) Filter the prepared medicinal solution and wait for subpackaging.
[0069] (5) Before dispensing, use sterile water for injection to adjust the dosage according to 2.0ml per vial, stopper and cap. After capping, the aluminum cove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com