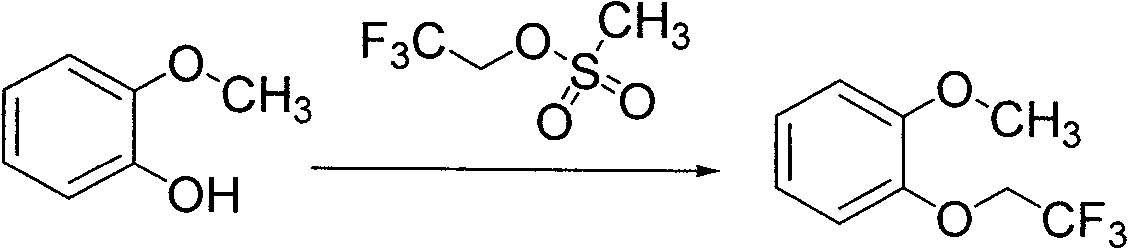
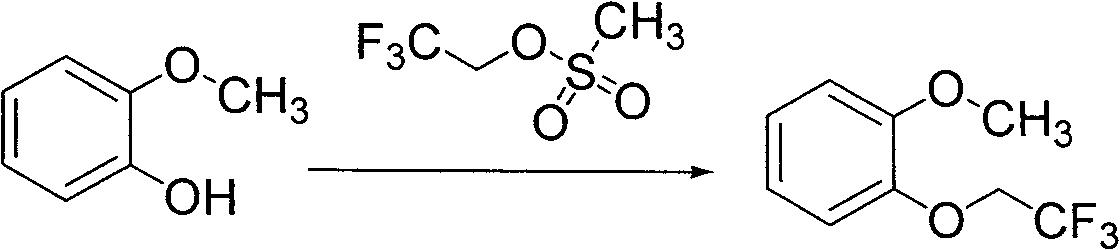
Preparation method of substituted phenyl methyl ether
A technology of phenyl methyl ether and dimethyl formamide, which is applied in the field of preparation of 2-phenyl methyl ether, can solve the problems of large environmental pollution, unsuitable for industrialized production, complicated purification process and the like, and achieves high safety, Good product quality and simple post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] In the there-necked flask equipped with mechanical stirring and reflux condenser, add 2-methoxyphenol (10g, 81mmol), dimethylformamide (100ml) and potassium carbonate (12.4g, 90mmol), stir, add 2, 2,2-Trifluoroethyl methanesulfonate (16g, 89mmol), stirred, heated to 150°C, maintained at reflux for 2 hours, stopped the reaction, filtered with suction, washed with dimethylformamide, combined the filtrates, concentrated under reduced pressure to dry (HPLC purity 96.8%), the residue was extracted three times with ether, the extracts were combined, washed twice with 10% sodium hydroxide, washed with water until neutral, and dried over anhydrous sodium sulfate. The desiccant was filtered off, and the filtrate was concentrated to dryness under reduced pressure to obtain 14.3 g of the product, with a yield of 86% (HPLC purity: 99.68%).
[0026] 1 H NMR (CDCl 3 ): 7.060~6.895, m, ArH; 4.418~4.355, t, CH 2 ;3.872,s,CH 3 .
Embodiment 2
[0028]
[0029] In the there-necked flask equipped with mechanical stirring and reflux condenser, add 2-methoxyphenol (10g, 81mmol), dimethylformamide (100ml) and sodium hydroxide (3.6g, 90mmol), stir, add 2 , 2,2-trifluoroethyl methanesulfonate (16g, 89mmol), stirred, heated to 100°C, maintained at reflux for 6 hours, stopped the reaction, filtered with suction, washed with dimethylformamide, combined the filtrates, concentrated under reduced pressure To dryness (HPLC purity 96.7%), the residue was extracted three times with ether, the combined extracts were washed twice with 10% sodium hydroxide, washed with water until neutral, and dried over anhydrous sodium sulfate. The desiccant was filtered off, and the filtrate was concentrated to dryness under reduced pressure to obtain 15.2 g of the product, with a yield of 91.5% (HPLC purity: 99.70%).
[0030] Elemental analysis (C 9 h 9 f 3 o 2 ): theoretical value (%) is C52.43, H 4.40, F27.65, O 15.52; experimental value (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com