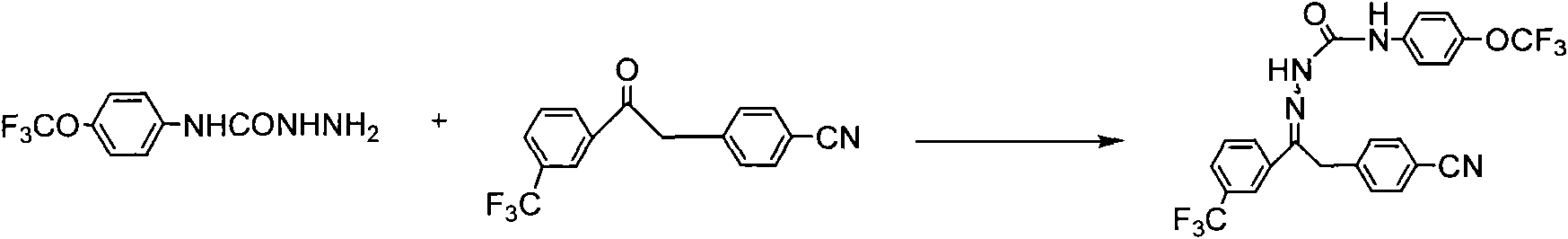
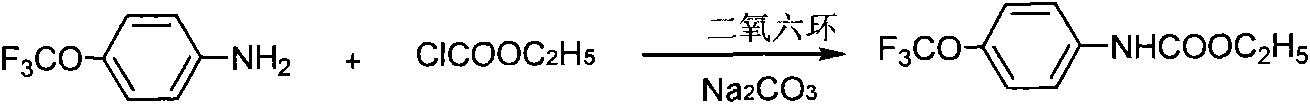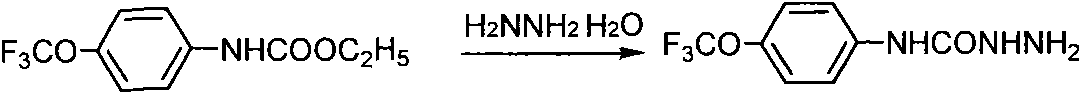Metaflumizone synthesis method
A technology of metaflumizone and its synthesis method, which is applied in the field of synthesis of metaflumizone pesticides, can solve the problems of difficult operation and economy, complex post-processing, high cost, etc., and achieve good economy, simple and practical preparation method, and low equipment corrosion small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the preparation of compound I
[0021] 1) Synthetic methyl p-trifluoromethoxyphenylcarbamate, synthetic route such as figure 1 .
[0022] In a 500ml three-necked bottle, equipped with a thermometer, a reflux condenser, and a constant pressure dropping funnel, add 26.55g of 4-trifluoromethylaniline, K 2 CO 3 27g and 300ml of dioxane, add 20ml of dioxane and 17.91g of ethyl chloroformate dropwise at room temperature, after the dropwise addition, heat to 50°C and stir for 6h, filter, wash the solid with 50ml of dioxane, The combined filtrates were spin-dried to obtain a white solid. Yield: 85%. The target product was detected by NMR.
[0023] 2) Synthetic p-trifluoromethylanilinohydrazide, the synthetic route is as follows figure 2 .
[0024] In a 500ml two-necked bottle, equipped with a thermometer and a reflux condenser, add 95g of 50% hydrazine hydrate and 35g of methyl p-trifluoromethoxyphenylcarbamate, heat to 90°C, keep warm for 25h, cool down t...
Embodiment 2
[0027] Embodiment 2: the preparation of compound I
[0028] 1) Synthetic methyl p-trifluoromethoxyphenylcarbamate, synthetic route such as figure 1 .
[0029] In a 500ml three-necked bottle, equipped with a thermometer, a reflux condenser, and a constant pressure dropping funnel, add 26.55g of 4-trifluoromethylaniline, K 2 CO 3 27g and 300mlTHF, add 20mlTHF and 17.91g ethyl chloroformate dropwise at room temperature. After the dropwise addition, heat to 50°C and stir for 6h, filter, wash the solid with 50mlTHF, combine the filtrates, and spin dry the liquid to obtain a white solid. Yield: 85%. The target product was detected by NMR.
[0030] 2) Synthetic p-trifluoromethylanilinohydrazide, the synthetic route is as follows figure 2 .
[0031] In a 500ml two-necked bottle, equipped with a thermometer and a reflux condenser, add 95g of 50% hydrazine hydrate and 35g of methyl p-trifluoromethoxyphenylcarbamate, heat to 90°C, keep warm for 25h, cool down to room temperature a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com