Application of Huperzine A in preparing medicaments
A technology of huperzine A and medicine, which is applied in the application field of huperzine A in pharmaceuticals, and achieves the effects of strong pharmacological effect, lowering eye pressure effect and good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
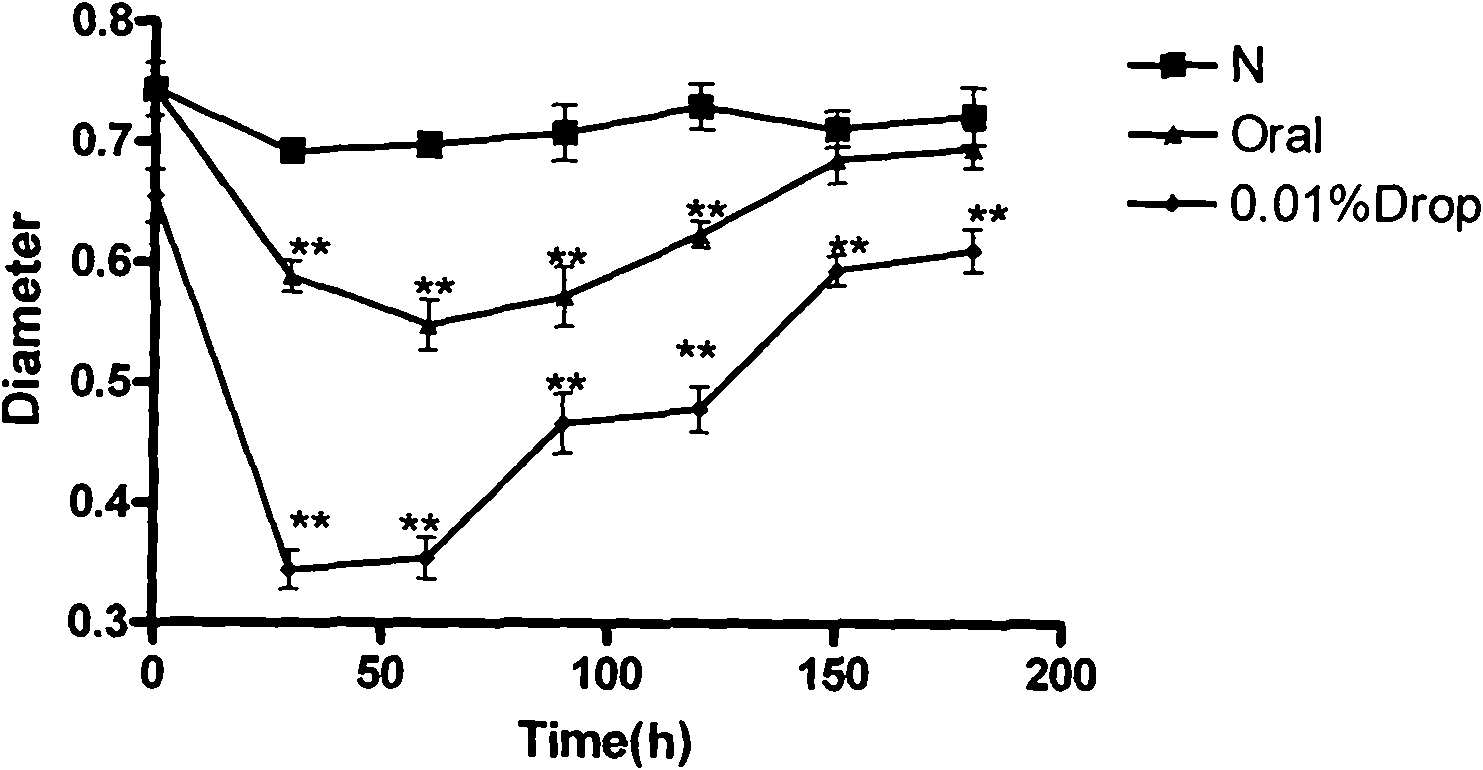
[0022] Embodiment one: the influence of huperzine A on the pupil diameter of rabbits
[0023] 1 Experimental materials
[0024] 1.1 Main Drugs and Reagents
[0025] Huperzine A (Institute of Materia Medica, Chinese Academy of Sciences, Shanghai).
[0026] 1.2 Test animals
[0027] New Zealand white rabbits, weighing 2.2-2.8kg, male and female, provided by the Experimental Animal Center of Shanghai Jiao Tong University School of Medicine, license number SYXK 2003-0026.
[0028] 2 Experimental method:
[0029] 2.1 Determination of the pupil
[0030] The rabbits were fixed in a rabbit box, and the pupil diameter of the rabbits was measured with a pupil ruler (Castroviejo caliper) under natural light.
[0031] 2.2 Administration method
[0032] 30 healthy white rabbits without eye diseases were selected and randomly divided into 3 groups (ten rabbits with 20 eyes in each group). Normal saline group: instill 50 μl of normal saline in each eye; huperzine A eye drop group: ins...
Embodiment 2
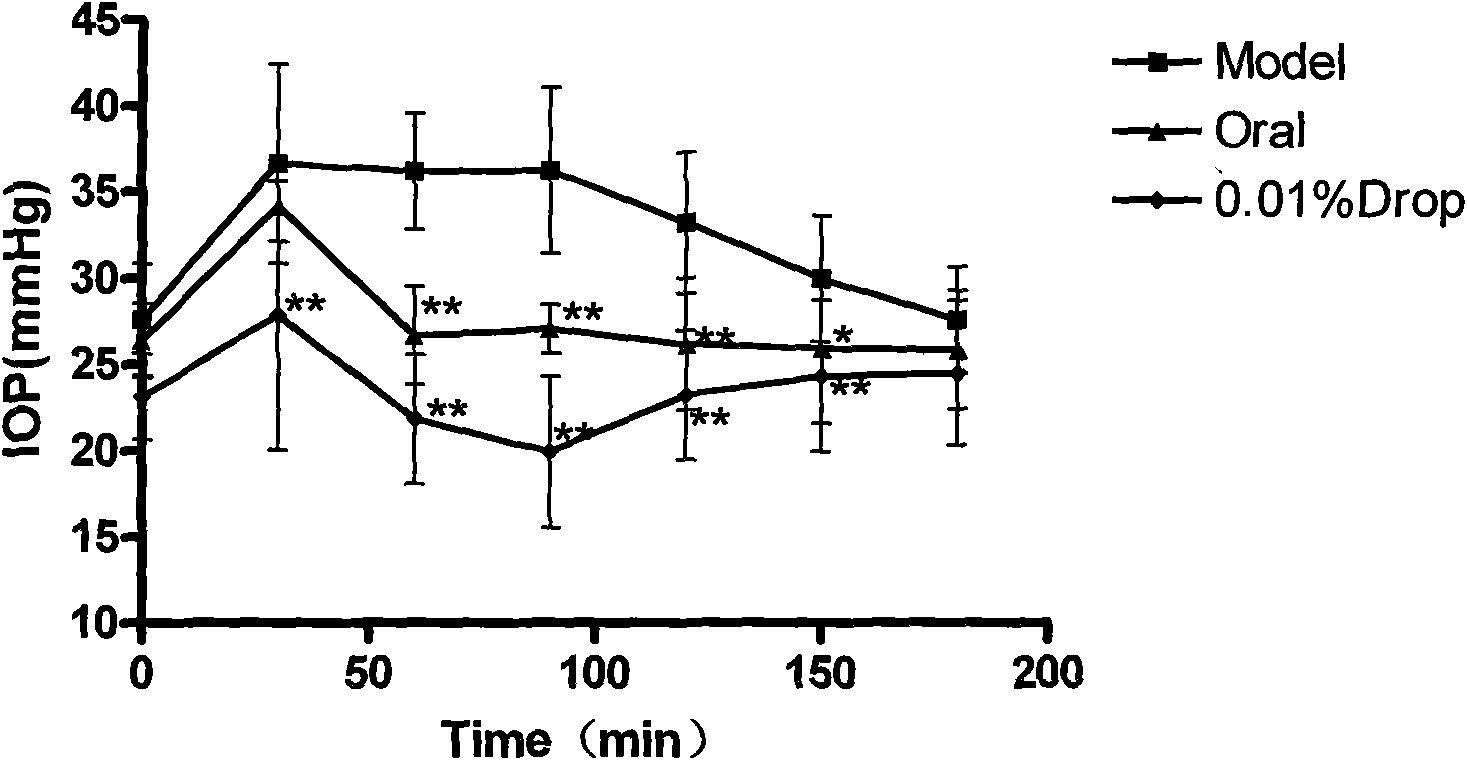
[0037] Example 2: Effect of huperzine A on intraocular pressure (IOP) in rabbits with high intraocular pressure induced by water load
[0038] 1 test animal
[0039] New Zealand white rabbits, weighing 2.2-2.8kg, male and female, provided by the Experimental Animal Center of Shanghai Jiao Tong University School of Medicine, license number SYXK 2003-0026.
[0040] 2 Experimental method:
[0041] 2.1 Tonometry
[0042] Fix the rabbit in a rabbit box, anesthetize the surface of the eyeball with 0.1% tetracaine, measure the intraocular pressure with a tonometer, repeat the measurement three times, take the average value, and record the intraocular pressure curve.
[0043] 2.2 Establishment of ocular hypertension model
[0044] water load induced ocular hypertension
[0045] Select healthy white rabbits without eye diseases, and inject 3% pentobarbital sodium (1 mL / kg) into each rabbit from the ear vein for general anesthesia. Both eyes were instilled with 1% tetracaine for lo...
Embodiment 3
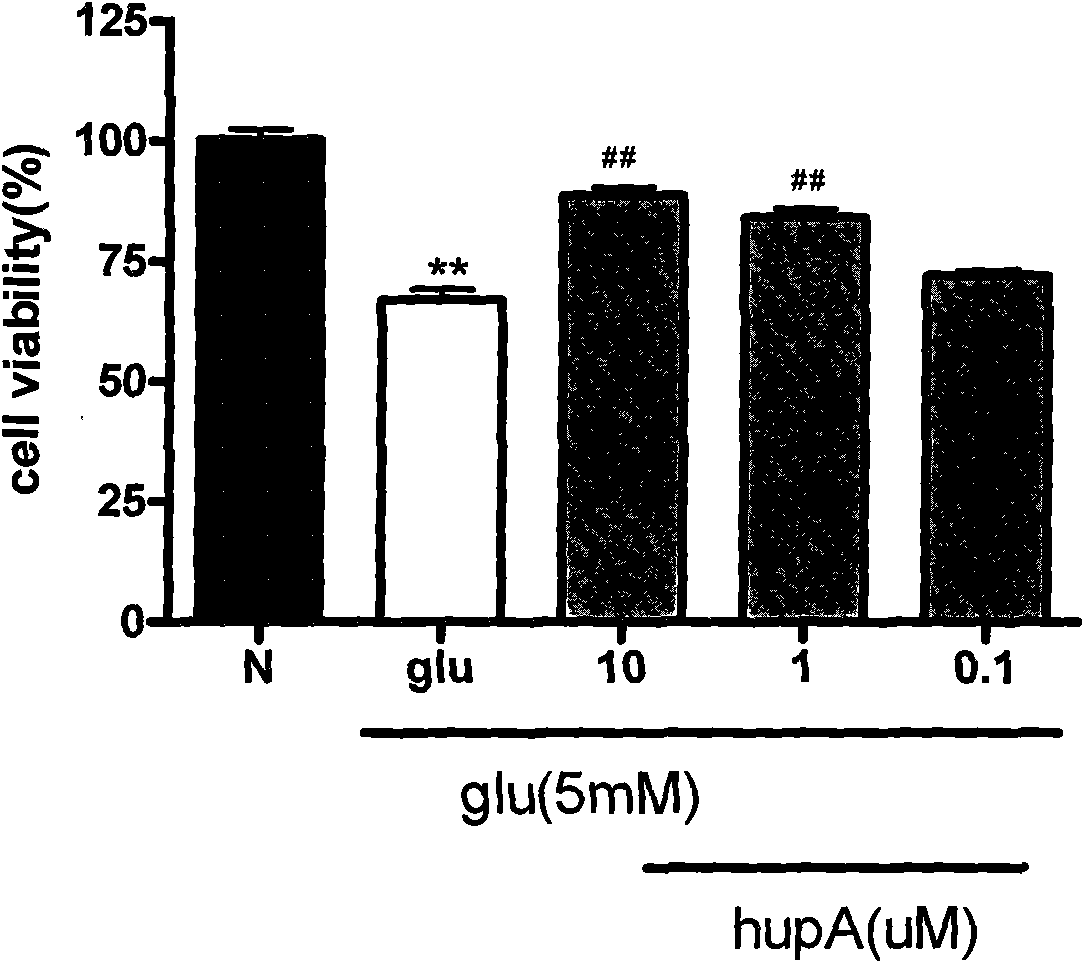
[0052] Example 3: Huperzine A protects retinal ganglion cells (RGC-5) in vitro
[0053] 1 Experimental materials
[0054] 1.1 Cell lines
[0055] RGC-5 retinal ganglion cell line was purchased from ATCC.
[0056] 1.2 Experimental drugs and reagents
[0057] Huperzine A (Shanghai Institute of Materia Medica, Chinese Academy of Sciences) was dissolved in DMSO to make a stock solution, stored at -20°C, and diluted to the required concentration with culture medium before use.
[0058] Glutamic acid (Glu) was purchased from Sigma, dissolved in culture medium to make a stock solution, stored at -20°C, and diluted to the desired concentration with culture medium before use.
[0059] 2 Experimental methods
[0060] Take the RGC-5 cells that grew stably and were cultured for 3-10 passages after recovery, digested with 0.25% trypsin and made cell suspension, and used 3×10 3 Inoculate in a 96-well plate, 100 μl per well. When the cells grew to 70%-80% confluence, the cells were div...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com