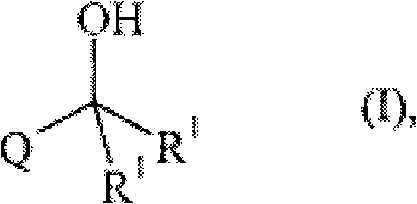Process for the production of tertiary alcohols
A technology of tertiary alcohol and alkyl group, applied in the field of producing tertiary alcohol, can solve problems such as unevenness, long activation of cerium trichloride, insoluble cerium trichloride, etc.
Inactive Publication Date: 2010-08-18
LONZA LTD
View PDF4 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In addition, the activation of cerium trichloride is lengthy and the activated cerium trichloride is not soluble in ethereal solvents such as tetrahydrofuran, which leads to heterogeneous reaction mixtures
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2-4
Embodiment 5
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
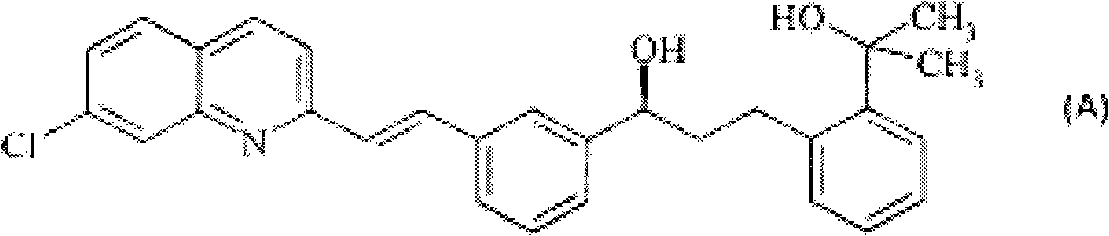
Tertiary alcohols are prepared by reacting carboxylic esters with Grignard reagents in ethereal solvents in the presence of lanthanum trichloride and lithium chloride. The method is particularly suitable for the production of (aS)-a-[3-[(1E)-2-(7-chloro-2-quino- linyl)ethenyl]phenyl]-2-(1-hydroxy-1-methylethyl)benzenepropanol of formula (A) which is an intermediate in the production of montelukast.
Description
technical field The present invention relates to the production method of the tertiary alcohol of formula (I) where R 1 for C 1-4 Alkyl, Q is C 1-10 Alkyl, C 2-10 Alkenyl, C 3-8 Cycloalkyl, aryl or heteroaryl or an organic moiety consisting of any two or more of the foregoing, C 1-10 Alkyl, C 2-10 Alkenyl, C 3-8 Each of cycloalkyl, aryl and heteroaryl is optionally replaced by one or more selected from hydroxyl, fluorine, chlorine, amino, C 1-4 Alkylamino and di(C 1-4 Alkyl) amino substituents are substituted. Background technique Tertiary alcohols with two lower alkyl groups on the methanolic carbon are valuable intermediates in the synthesis of a variety of pharmaceutically active compounds. For example, (αS)-α-[3-[(1E)-2-(7-chloro-2-quinolyl)vinyl]phenyl]-2-(1-hydroxyl-1-methyl Ethyl)-phenylpropanol is known as montelukast (1-[[[(1R)-1-[3-[(1E)-2-(7-chloro-2-quinolinyl)vinyl]phenyl]-3- [2-(1-Hydroxy-1-methylethyl)phenyl]propyl]sulfur]methyl]cyclopropaneacet...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07B49/00C07C29/147C07C31/135C07C31/12C07D215/18
CPCC07B49/00
Inventor 约翰·麦克加瑞堤弗朗西斯·迪周周
Owner LONZA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com