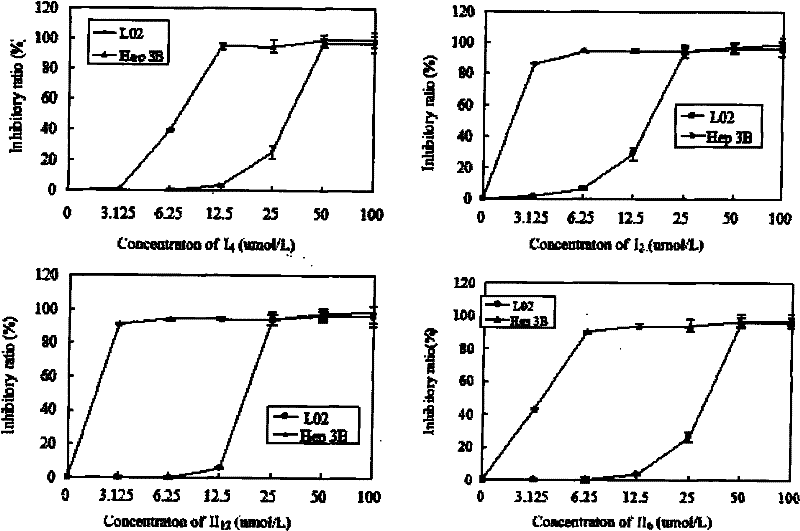
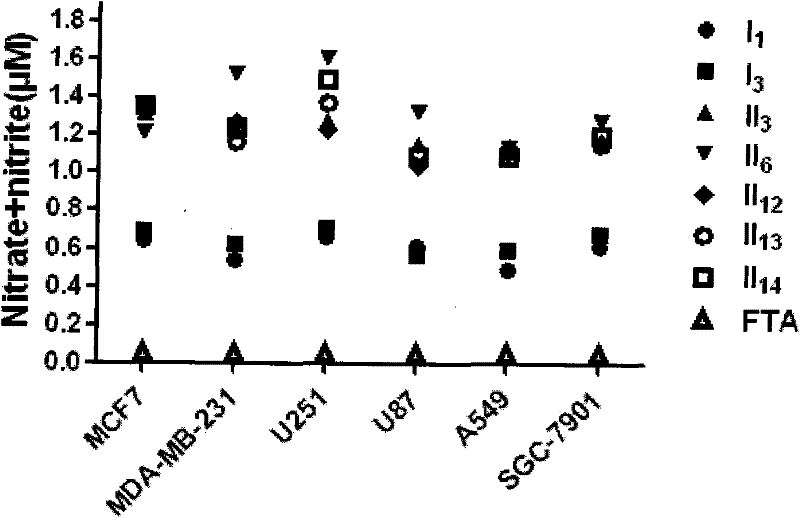
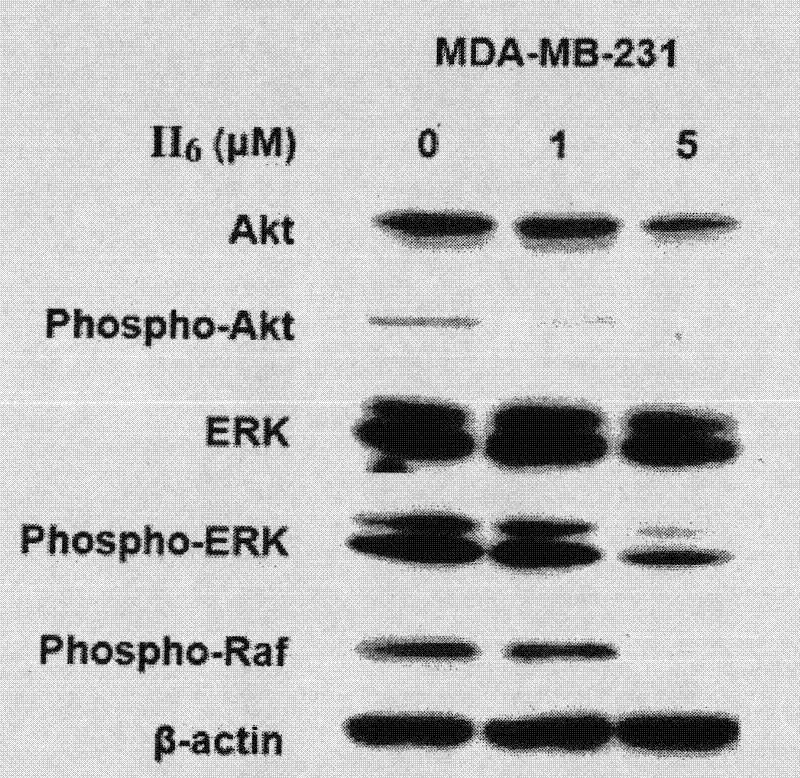
Nitric oxide donor-type farnesyl thiosalicylic acid derivative, and preparation method and medical application thereof
A kind of technology of farnesyl thiosalicylic acid and derivatives, which is applied in the application field of preparing antitumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Preparation of 2-[(4-benzenesulfonyl-5-oxo-1,2,5-oxadiazole-3-)oxy]ethylamine (1a)
[0160] Dissolve 3mL (50mmol) ethanolamine and 1.85g (5mmol) 2-oxo-3,4-diphenylsulfonyl-1,2,5-oxadiazole in 20mLTHF, cool in an ice bath, and drop into 2.5mol / L NaOH solution 2 mL, react at room temperature for 0.5 h, add 1 mL of 2.5 mol / L NaOH solution, continue to stir until the raw materials are completely reacted, pour 80 mL of water, extract with dichloromethane (3×20 mL), wash with saturated brine, dry over anhydrous sodium sulfate, and concentrate , recrystallized from methanol / water to obtain 0.93 g of white solid (1a), yield 66%, mp: 102-104°C.
[0161] N-{2-[(4-Benzenesulfonyl-5-oxo-1,2,5-oxadiazole-3-)oxy]ethyl}farnesylthiosalicylic acid amide (I 1 ) preparation
[0162] Dissolve 18.0 g (0.50 mmol) FTA and 14.0 mg (0.65 mmol) DCC in 15 mL anhydrous CH 2 Cl 2 , stirred at room temperature for 30 minutes, added 0.18g (0.60mmol) 1a and a catalytic amount of DMAP, reacted at r...
Embodiment 2
[0164] Preparation of N-{[2-(4-Benzenesulfonyl-5-oxo-1,2,5-oxadiazole-3-)oxy]ethoxy}piperazine (1b)
[0165] Referring to the preparation method of 1a, a light yellow solid was obtained from hydroxyethylpiperazine with a yield of 55%, mp: 90-92°C.
[0166] 3-{4-[3-[(4-Benzenesulfonyl-5-oxo-1,2,5-oxadiazole-3-)oxy]propoxy]-1,4-dioxobutoxy }farnesyl methyl thiosalicylate (I 2 ) preparation
[0167] N-{[2-(4-Benzenesulfonyl-5-oxygen-1,2,5-oxadiazole-3-)oxygen]ethoxy}piperazinylfarnesylthiosalicylic acid imide ( I 2 ) preparation
[0168] Refer to I 1 The preparation method is prepared by the reaction of FTA and 1b, a colorless transparent oil, and the yield is 61%. IR (KBr, cm -1 ) v: 2926, 1731, 1648, 1551, 1454, 1371, 1167; 1 H NMR (CDCl 3 , 300MHz): δ8.05(d, 2H, J=7.8Hz, Ar-H), 7.75(m, 1H, Ar-H), 7.61(m, 2H, Ar-H), 7.38(m, H, Ar-H), 7.28(m, 3H, Ar-H), 5.28(m, 1H, SCH 2 C H ), 5.08(m, 2H, 2×CH 2 C H =CCH 3 ), 4.58(t, 2H, J=5.1Hz, C H 2 O), 3.84(d, 2H, J=4.5Hz, N...
Embodiment 3
[0170] Preparation of 4-[(4-benzenesulfonyl-5-oxo-1,2,5-oxadiazole-3-)oxy]-2-butyn-1-ol (1c)
[0171] Referring to the preparation method of 1a, a white solid was obtained from 2-butynediol with a yield of 63%, mp: 110-112°C.
[0172] {4-[(4-Benzenesulfonyl-5-oxo-1,2,5-oxadiazol-3-)oxy]-2-ynyl}farnesyl butyl thiosalicylate (I 3 ) preparation
[0173] Refer to I 1 The preparation method is prepared by the reaction of FTA and 1c, a colorless transparent oil, the yield is 65%, IR (KBr, cm -1 ) v: 2934, 1724, 1618, 1548, 1453, 1357, 1167; 1 H NMR (CDCl 3 , 300MHz): δ8.06(d, 2H, J=7.8Hz, Ar-H), 8.01(d, 1H, J=7.8Hz, Ar-H), 7.74(t, 1H, J=7.2Hz, Ar -H), 7.62(m, 2H, Ar-H), 7.47(t, 1H, Ar-H), 7.33(m, 1H, Ar-H), 7.20(m, 1H, Ar-H), 5.33( m, 1H, SCH 2 C H ), 5.12(m, 4H, 2×CH 2 C H =CCH 3 , COOCH 2 ), 5.00(s, 2H, OCH 2 ), 3.60 (d, 2H, J=7.2Hz, SC H 2 ), 1.88-2.04 (m, 8H, 2×CHC H 2 C H 2 CH), 1.48-1.68 (m, 12H, 4×CH=CC H 3 ); ESI-MS (m / z): 651 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com