4-Substituted anilino epipodophyllotoxin derivatives and their uses
An epipodophyllotoxin and anilino-based technology, which is applied in the field of 4-substituted anilino epipodophyllotoxin derivatives and applications, and can solve problems such as poor water solubility, bone marrow suppression, and easy drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
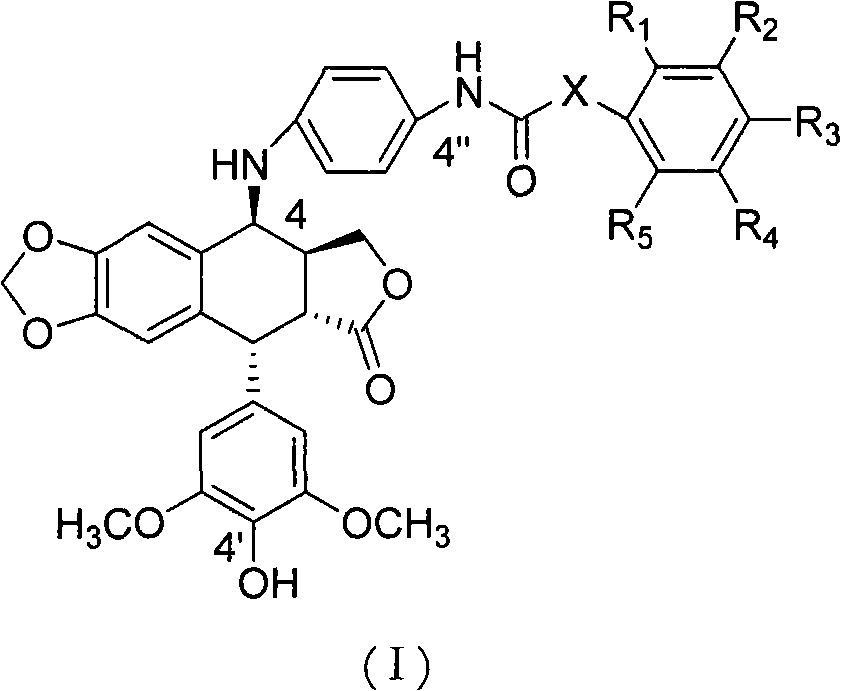
[0013] Embodiment 1 The synthetic general formula of the present invention is:
[0014]
Embodiment 2
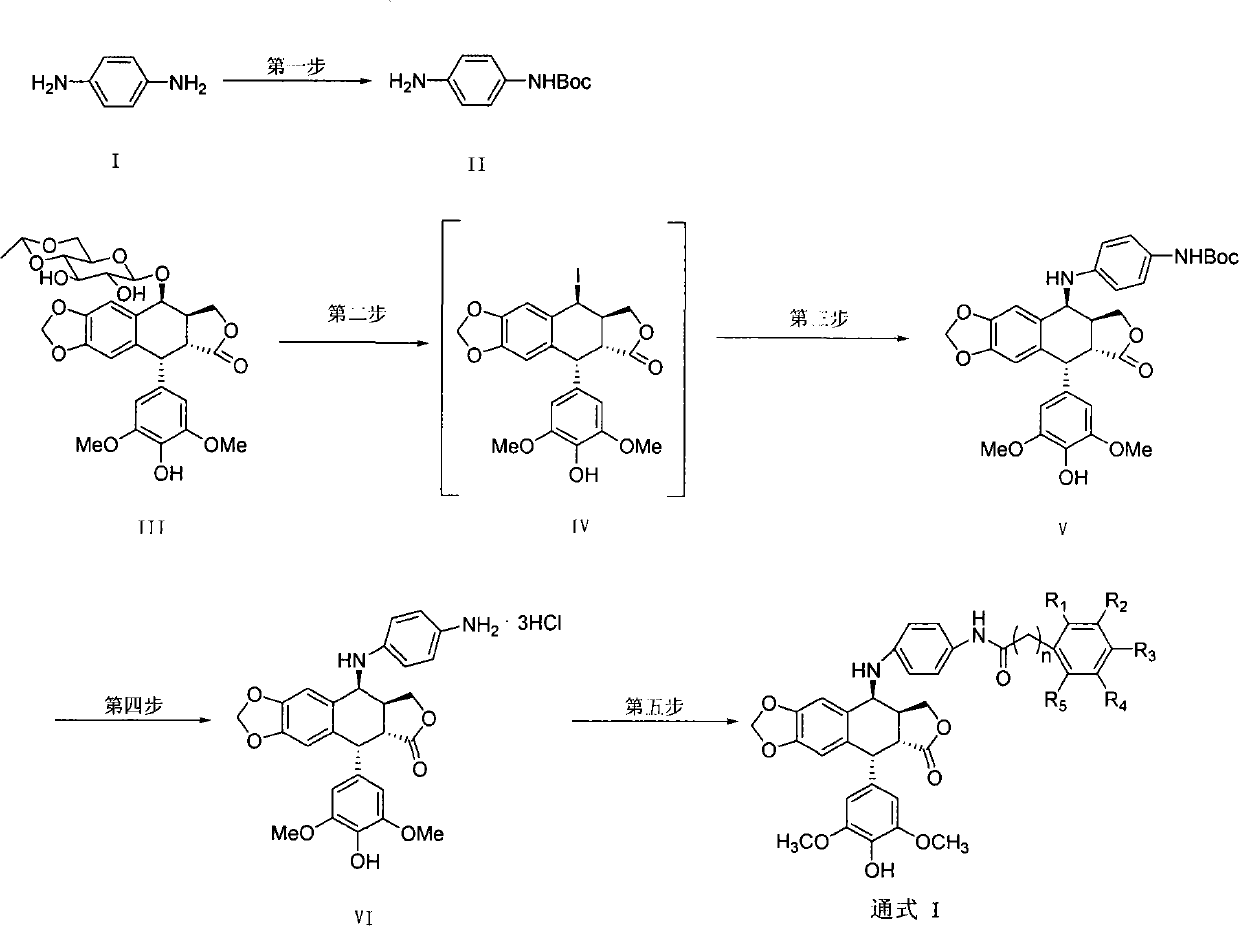
[0015] Example 2: Preparation of compound 4'-O-desmethyl-4-deoxy-[4-(4"-p-methoxybenzamido)anilino]epipodophyllotoxin (GL1)
[0016] first step: (Refer to literature method: Francesco Troisi et al. Tetrahedron Letters 2007, 48, 7986-7989)
[0017] 5.05g di-tert-butyl dicarbonate (Boc 2 (2, 23mmol) was dissolved in dioxane, under ice-bath conditions, was added dropwise in the dioxane solution of 5g p-phenylenediamine (compound 1, 46.3mmol), stirred overnight at room temperature after the dropwise addition, Then the reaction solution was concentrated under reduced pressure, and the remaining residue was purified by silica gel chromatography (eluent: ethyl acetate / petroleum ether=7 / 3) to obtain compound II, yield: 92.7%; yellow solid, melting point: 108.9 -113.7°C.
[0018] Step two:
[0019] Under nitrogen protection, 5g etoposide (compound III, 8.5mmol) and 5.11g sodium iodide (34mmol) were dissolved in 50mL anhydrous acetonitrile; another dry 4.3mL trimethylchlorosilane ...
Embodiment 3
[0026] Example 3: Preparation of compound 4'-O-desmethyl-4-deoxy-[4-(4"-p-fluorobenzamido)anilino]epipodophyllotoxin (GL2)
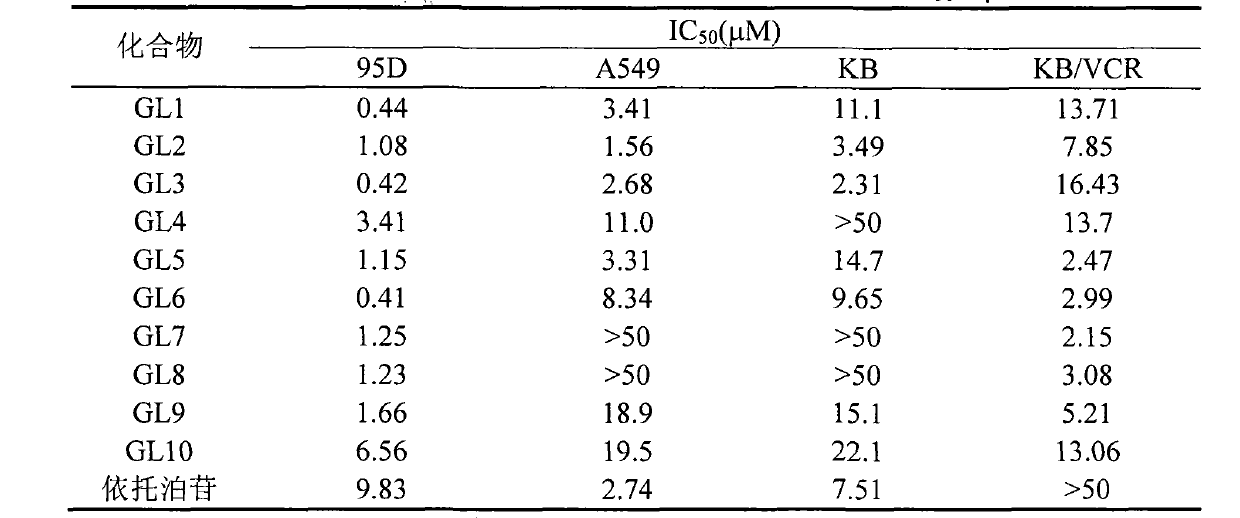
[0027] The operating process is as in Example 1, except that p-fluorobenzoic acid is used instead of p-methoxybenzoic acid. A yellow solid was obtained, yield: 57%, melting point: 157.2-158.9°C, 1 HNMR (500MHz, CDCl 3)δ7.86 (m, 2H, 2"', 6"'-H), 7.66 (s, 1H, -CONH-), 7.44 (d, J=8.5Hz, 2H, 3", 5"-H) , 7.14(t, J=8.5Hz, 3"', 5"'-H), 6.76(s, 1H, 5-H), 6.55(d, J=9.0Hz, 2H, 2", 6"-H ), 6.52 (s, 1H, 8-H), 6.34 (s, 2H, 2', 6'-H), 5.95 and 5.97 (AB q, J=1.0Hz, 2H, -OCH 2 O-), 4.66(m, 1H, 4-H), 4.59(d, J=5.5Hz, 1H, 1-H), 4.36(t, J=8.0Hz, 1H, 11-H), 3.98(t , J=8.0Hz, 1H, 11-H), 3.85 (br, 1H, -CONH), 3.79 (s, 6H, 3', 5'-OCH 3 ), 3.13(dd, J=5.0Hz, 14.0Hz, 1H, 2-H), 3.00(m, 1H, 3-H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com