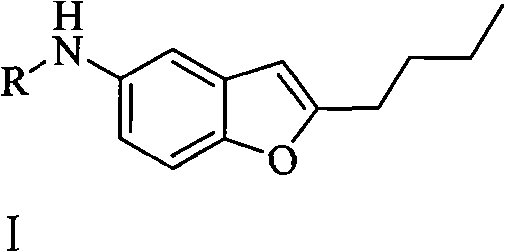
2-n-butyl-5-substituted aminobenzofuran and its preparation method
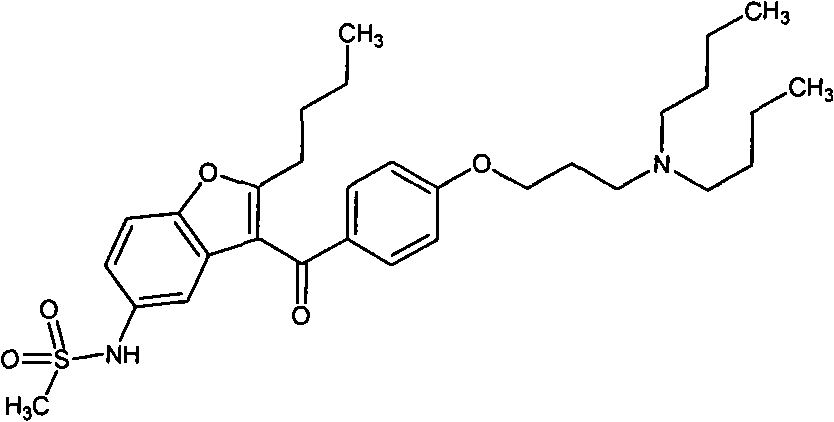
A technology of benzyloxycarbonyl and compound, applied in the key intermediate of dronedarone and its preparation field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
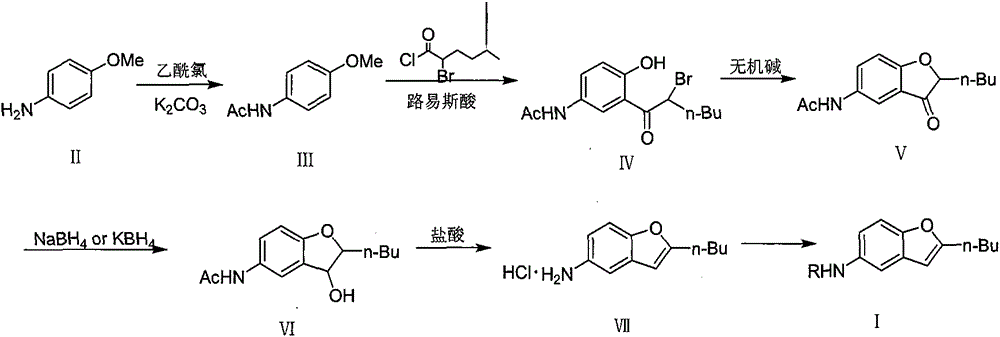
[0069] Example 1: Preparation of 4-acetamidoanisole (compound III).
[0070]
[0071]In a 10L three-necked flask, under nitrogen protection, add 615g (4.99mol) of p-aminoanisole, 5L of dichloromethane and 690g (4.86mol) of potassium carbonate, and then reduce the reaction system to -10~-5°C, and 470g ( 5.99mol) 1.5L dichloromethane solution of acetyl chloride was added dropwise into the reaction system, reacted at -10~-5°C for 1h, raised to room temperature, stirred for 17 hours, then stopped stirring, left the reaction solution standing, and the upper layer liquid Pour into ice water, adjust pH=8~9 with sodium carbonate, separate liquid, extract water phase with dichloromethane, combine organic phase, dry with anhydrous sodium sulfate, filter, concentrate to remove part of solvent, add n-hexane during stirring , precipitated a white solid, and dried to obtain 595.1 g of a white solid (Ⅲ), yield: 65%.
Embodiment 2
[0072] Example 2: Preparation of 2-(2-bromo-n-hexanoyl)-4-acetamidophenol (compound IV).
[0073]
[0074] In a 100ml three-necked flask, under nitrogen protection, add 4.1g (0.025mol) of 4-acetamidoanisole (compound III), 35ml of dichloromethane, then drop the reaction system to 0°C, and add 8.0g (0.105mol) of Add aluminum trichloride in batches, react for 0.5 hours, then drop to 0°C, add dropwise 10.7g (0.05mol) 2-bromo-n-hexanoyl chloride in 30ml dichloromethane solution, then raise the temperature of the system to reflux, stir and reflux overnight. Pour the reaction solution into ice water, extract and separate the liquids with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate under reduced pressure, add n-hexane during stirring, and precipitate a yellow solid, dry it to obtain a yellow solid ( Ⅳ) 7.5 g, yield: 91%. The melting point is 137-138°C.
Embodiment 3
[0075] Example 3: Preparation of 2-(2-bromo-n-hexanoyl)-4-acetamidophenol (compound IV)
[0076]
[0077] Under nitrogen atmosphere, in a 500ml three-necked flask, add 46.5g (0.218mol) 2-bromo-n-hexanoyl chloride, 240ml dichloromethane, stir and cool the reaction system below 0°C, add 30g (0.182mol) 4-acetamidobenzene Methyl ether (compound Ⅲ), stirred for 0.5h. Keeping at 0°C, 60.7g (0.44mol) of aluminum trichloride was added in portions. Then the system was warmed up to reflux, stirred and refluxed overnight. Pour the reaction solution into ice water, extract and separate the liquids with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate under reduced pressure, add n-hexane during stirring, and precipitate a yellow solid, dry it to obtain a yellow solid ( Ⅳ) 45.9g, yield: 77%. The melting point is 137-138°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com