Somatostatin analogue for detecting cancers as well as preparation method and application thereof
A technology of somatostatin and analogues, which is applied in the field of biomedicine, can solve the problem of no suggested cancer markers, etc., and achieve intuitive and accurate labeling results, accurate results, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
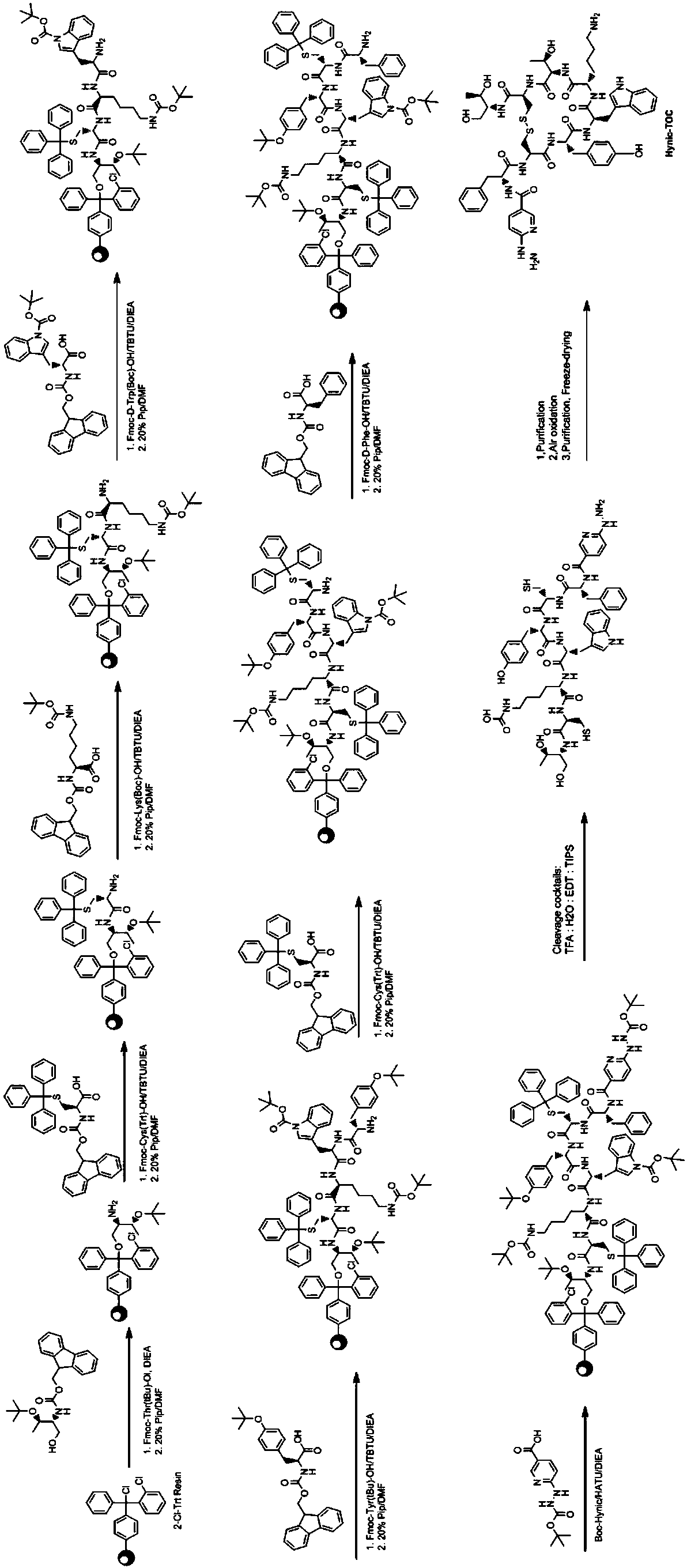
[0044] The somatostatin analog of the above-mentioned structure of the present invention is preferably synthesized by a 9-fluorenylmethoxycarbonyl solid-phase synthesis method, and the solid-phase synthesis method comprises the following steps: making the solid-phase synthesis resin Fmoc-Thr(tBu)-Ol-2-Cl -After Trtresin swells, wash and remove Fmoc to obtain de-Fmoc resin; Fmoc-Cys(Trt)-OH, Fmoc-Thr(tbu)-OH, Fmoc-Lys(Boc)-OH, Fmoc-D-Trp(Boc) )-OH, Fmoc-Tyr(tbu)-OH, Fmoc-Cys(Trt)-OH, Fmoc-D-Phe-OH and Boc-HYNIC are coupled to the de-Fmoc resin to form a peptide bond, and the reaction in the resin is collected The obtained polypeptide is oxidized to obtain the somatostatin analog.
[0045] First, the solid-phase synthetic resin Fmoc-Thr(tBu)-Ol-2-Cl-Trtresin is swollen, washed and removed from Fmoc to obtain the de-Fmoc resin. Said swelling and washing preferably use dimethylformamide. The present invention preferably reacts with resin with the volume concentration of 25% pipe...
Embodiment 1
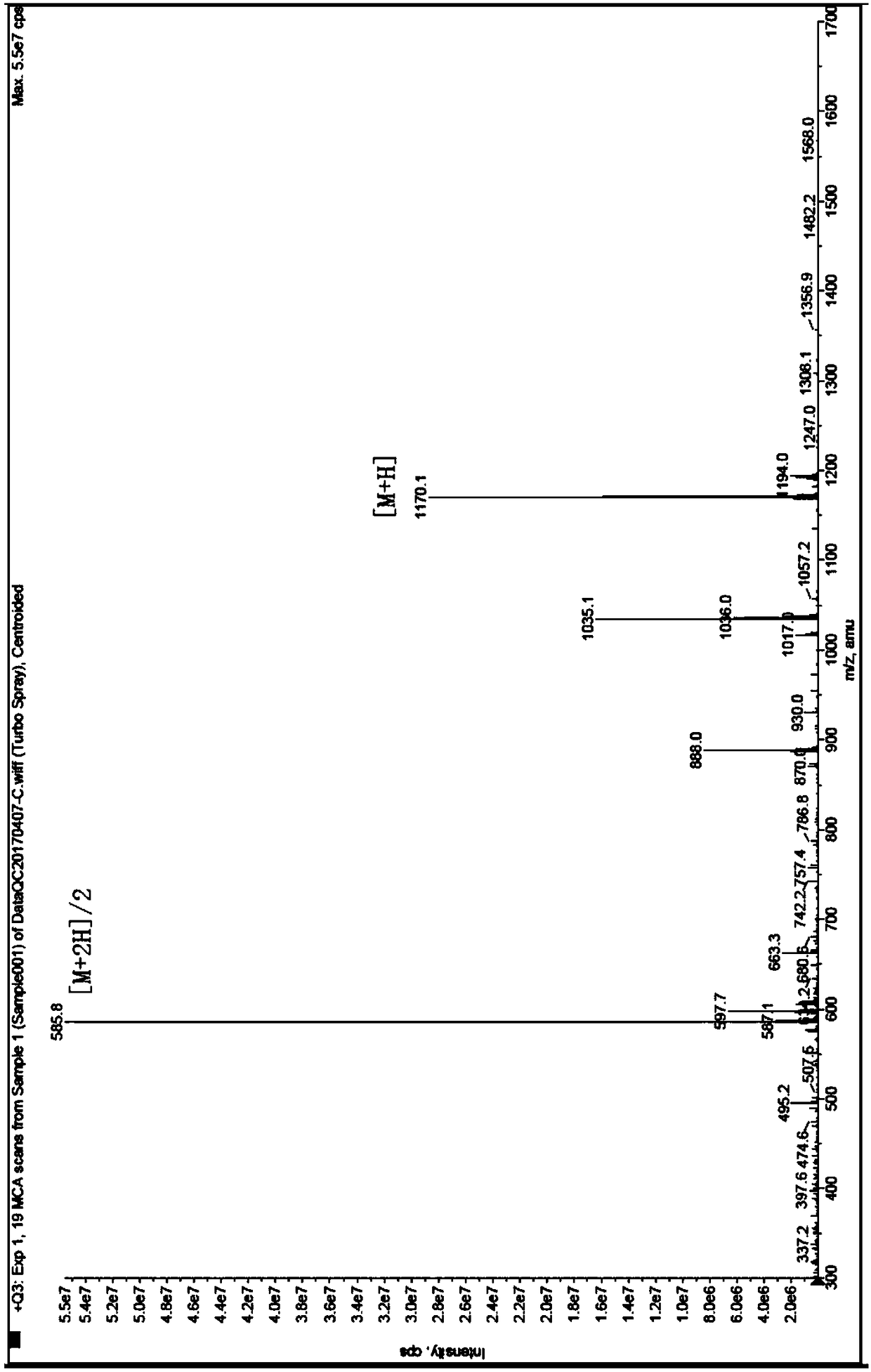
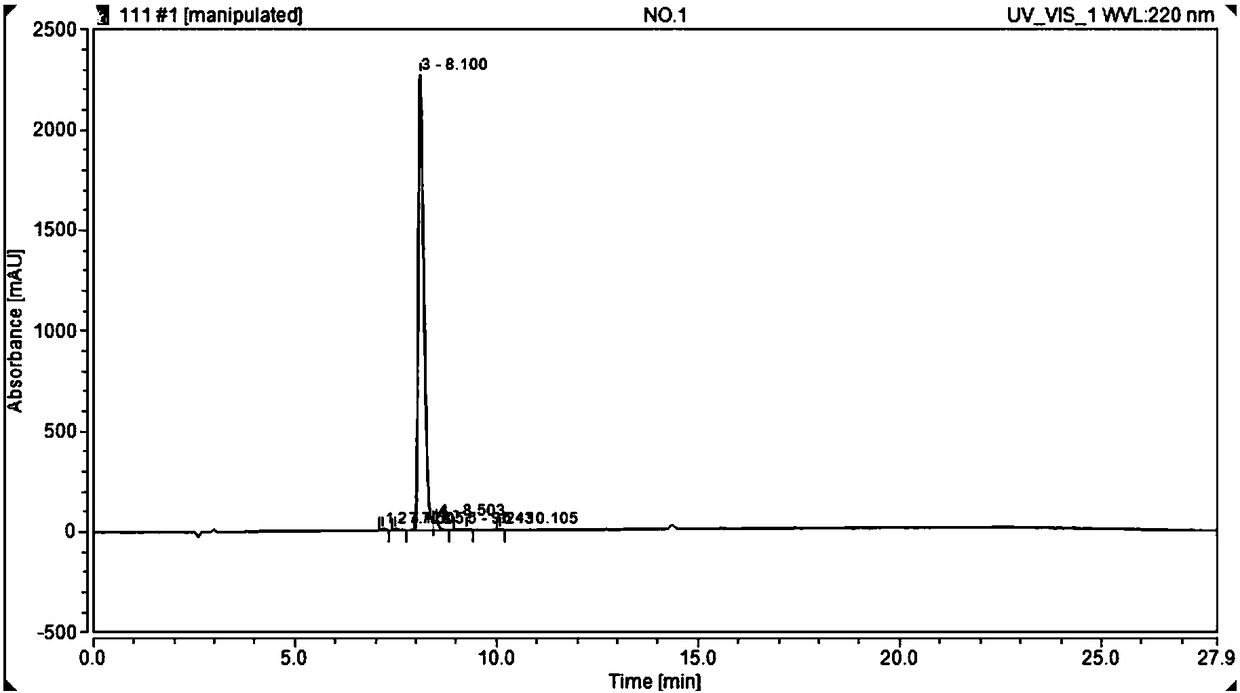
[0057] Synthesis and Purification of Polypeptide HYNIC-TOC
[0058] 1. Main reagents:
[0059] Fmoc-Thr(tBu)-Ol-2-Cl-Trt resin(0.3-0.6mmol / g), Fmoc-Cys(Trt)-OH, Fmoc-Thr(tbu)-OH, Fmoc-Lys(Boc)-OH , Fmoc-D-Trp(Boc)-OH, Fmoc-Tyr(tbu)-OH, Fmoc-D-Phe-OH, Boc-HYNIC, HBTU, HOBt, diisopropylethylamine (DIEA), piperidine ( PIP), dimethylformamide (DMF), dichloromethane (DCM), methanol (MeOH), anhydrous methyl tert-butyl ether (MTBE), trifluoroacetic acid (TFA), triisopropylsilane (TIPS ), ethanedithiol (EDT), acetonitrile (ACN), pure water (H 2 O), phosphate buffered saline (PBS), Kaiser reagent.
[0060] Second, the main equipment:
[0061] Refrigerated centrifuge (Shanghai Luxiang TDZ5-WS), preparative high performance liquid chromatography (Chuangxin Tongheng LC6000), analytical high performance liquid chromatography (Thermo Fisher Ultimate 3000), mass spectrometer (Waters ZQ-2000), freeze-drying machine (Boyikang FD-1).
[0062] Three, the synthesis process (see figure 1 ):...
Embodiment 2
[0097] The HYNIC-TOC prepared in Example 1 was sent to Midu (Nanjing) Biotechnology Co., Ltd. 99m Tc mark, get 99m Tc-HYNIC-TOC. right 99m The stability of Tc-HYNIC-TOC was determined
[0098] 1. Quality control and solvent stability testing
[0099] Using the Radio-iTLC method for 99m The radiochemical purity of Tc-HYNIC-TOC was tested, the support was silica gel impregnated glass fiber strips, and the developing agents were 50% acetonitrile and 0.5M citric acid / sodium citrate buffer (pH=5).
[0100] Utilize Radio-iTLC pair 99m Tc-HYNIC-TOC conducts quality control and in vitro solvent stability testing, and the results show that:
[0101] 99m The quality control of Tc-HYNIC-TOC (L17091801 and L17091901) was qualified, and the radiochemical purity (RCP) was 100%, meeting the test requirements.
[0102] 2. In vitro stability of the test object
[0103] 99m Tc-HYNIC-TOC was stored in 0.2M PB buffer (pH=6) at room temperature, its radiochemical purity was detected by R...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com