Functionalized ionic liquid containing a phosphorus and oxygen structure, and preparation method and application thereof
A technology of ionic liquid and anion, which is applied in the preparation of the aforementioned ionic liquid, the application of uranium in the extraction of uranium-containing water system, and the field of functionalized ionic liquid, which can solve the problems that hinder the smooth progress of the extraction process.
Inactive Publication Date: 2010-09-22
BEIJING RES INST OF CHEM ENG & METALLURGY
View PDF0 Cites 32 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
With conventional alkylimidazole hydrophobic room temperature ionic liquids such as [C 8 mim][PF 6 ] or [C 4 mim][PF 6 ] as a diluent, compounded with uranium extractant tributyl phosphate (TBP) to extract uranium in uranium-containing water system, which can obtain higher extraction efficiency ([2]P.Giridhar, K.A.Venkatesan, T.G.Srinivasan. P.R.Vasudeva Rao, Journal of Radioanalytical and Nuclear Chemistry, 2005, 265, 31-38), but there is a problem that the extraction system produces three phases, which hinders the smooth progress of the extraction process
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
Login to View More
Abstract
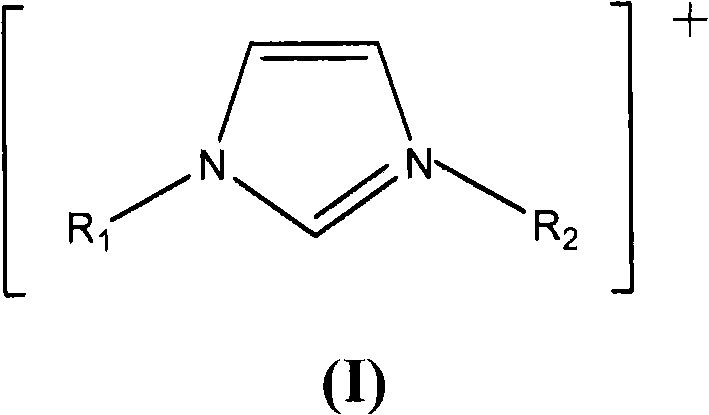
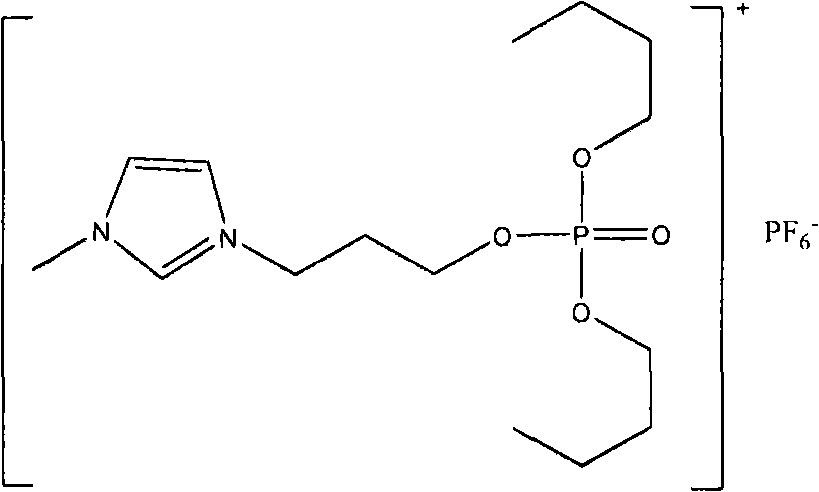
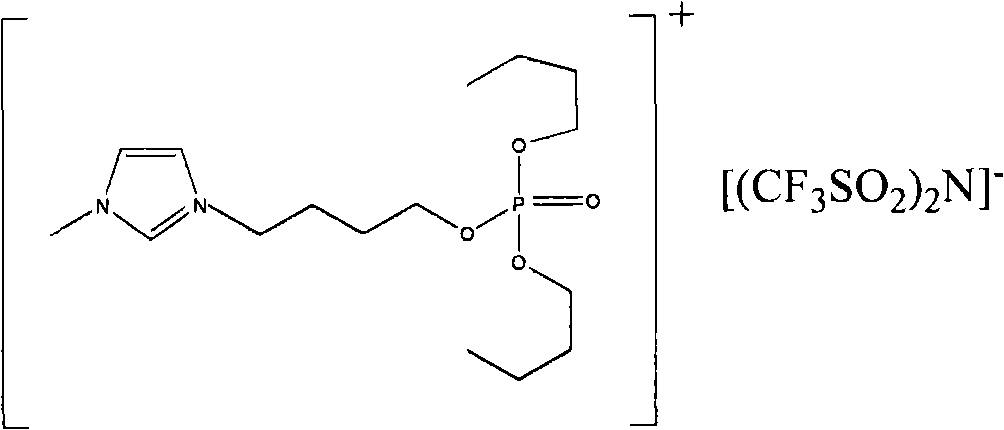
The invention relates to functionalized ionic liquid containing a phosphorus and oxygen structure with a general formula of A+ B-, which is characterized in that A+ is shown in a formula (I), and R 1 and R 2 are defined in the specification; and B- is Cl-, Br-, I -, BF4-, PF6-, NO3-, CF3SO3-, [(CF3SO2) 2N] - or CH3CO2-. The invention also relates to a method for preparing ionic liquid with the formula of A + B-, which comprises the following steps: a compound in a formula (III) is reacted with a compound in a formula (II ') to obtain a compound in a formula (II'') , and then a compound in a formula (II'') is reacted with a compound in a formula (IV) to obtain a halide ionic liquid A + B-, wherein B- is Cl-, Br-or I-, and ionic liquids with the other anions can be obtained by carrying out the ion exchange of the halide ionic liquid. The invention also relates to application of the ionic liquids in extraction of uranium from uranium-containing water systems. HO-(CH2) nCH2-X (III).
Description
technical field The invention relates to an imidazole-type ionic liquid, in particular to a functionalized ionic liquid (TSILs, Task Specific Ionic Liquids or FILs, Functionalized Ionic Liquids) containing phosphorus and oxygen structures. The present invention also relates to the preparation method of the aforementioned ionic liquid and the application of the ionic liquid, especially the application of extracting uranium in the uranium-containing water system. Background technique Compared with traditional organic solvents and electrolytes, ionic liquids have a series of outstanding advantages: (1) almost no vapor pressure, non-volatile, colorless, and odorless; (2) have a larger stable temperature range and better chemical stability (3) The solubility of inorganic substances, organic substances and polymers can be adjusted through the design of anions and cations. Due to the above excellent properties, it is regarded as a "green" solvent, which can replace the conventiona...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07F9/6506C22B3/38C22B60/02
Inventor 李宏宇刘浩窦军彦刘正平
Owner BEIJING RES INST OF CHEM ENG & METALLURGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com