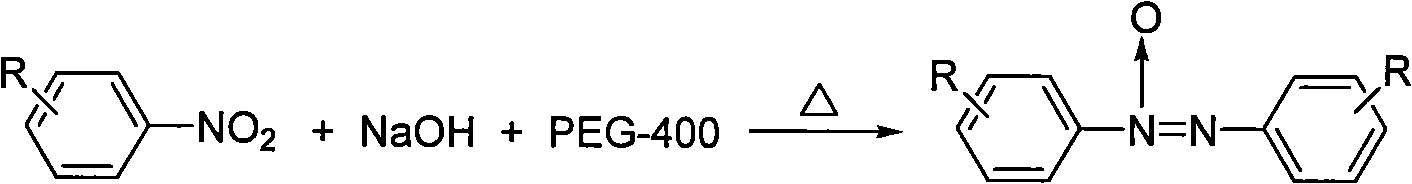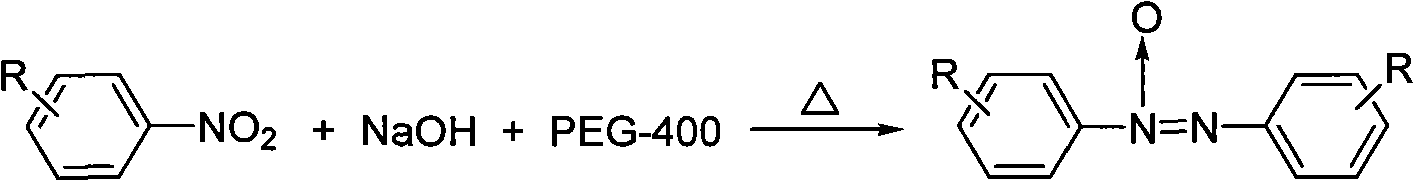Preparation method of azoxybenzene compound
A technology for oxidizing azobenzene and compounds, applied in the direction of organic chemistry, etc., can solve the problems of complicated operation, high cost, corrosion of concentrated alkali equipment, etc., and achieve the effects of reducing environmental pollution, reducing reaction cost and short reaction period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the preparation of azobenzene oxide
[0018] 1. At room temperature, add 0.25g (2mmol) nitrobenzene, 0.4g (10mmol) solid NaOH and 4mL PEG-400 into a reaction flask equipped with an electromagnetic stirrer, a reflux condenser and a thermometer, then stir and heat up to 90°C Left and right reactions, traced by thin layer chromatography until the disappearance of raw materials, took 1h;
[0019] 2. Stop the reaction, dilute with water, place, filter with suction, and dry at room temperature to obtain a crude product;
[0020] 3. Recrystallized with absolute ethanol to obtain azobenzene oxide as a yellow solid with a yield of 85%, m.p.35-36°C, 1 HNMR (CDCl 3 , 300MHz) δ: 7.40-7.54 (m, 6H), 8.05-8.19 (t, 2H), 8.30-8.32 (d, 2H).
Embodiment 2
[0021] Embodiment 2: the preparation of 3,3'-azobenzene dichloride
[0022] 1. At room temperature, add 0.32g (2mmol) 3-chloronitrobenzene, 0.2g (5mmol) solid NaOH and 4mL PEG-400 into a reaction flask equipped with an electromagnetic stirrer, a reflux condenser and a thermometer, and then stir to raise the temperature Reaction at about 90°C, followed by thin-layer chromatography until the disappearance of the raw materials, took 1h;
[0023] 2. Stop the reaction, dilute with water, place, filter with suction, and dry under normal pressure to obtain a crude product;
[0024] 3. Recrystallized with absolute ethanol to obtain 3,3'-dichloroazobenzene oxybenzene as a light yellow solid with a yield of 90%, m.p.96-97°C, 1 HNMR (CDCl 3 , 300MHz) δ: 7.40-7.57 (m, 4H), 7.99-8.21 (m, 2H), 8.26-8.32 (m, 2H).
Embodiment 3
[0025] Embodiment 3: the preparation of 3,3'-azobenzene dichloride
[0026] 1. At room temperature, add 0.32g (2mmol) 3-chloronitrobenzene, 0.4g (10mmol) solid NaOH and 4mL PEG-400 into a reaction flask equipped with an electromagnetic stirrer, a reflux condenser and a thermometer, then stir to raise the temperature Reaction at about 90°C, followed by thin-layer chromatography until the disappearance of raw materials, took 0.5h;
[0027] 2. Stop the reaction, dilute with water, place, filter with suction, and dry under normal pressure to obtain a crude product;
[0028] 3. Recrystallized with absolute ethanol to obtain 3,3'-dichloroazobenzene oxybenzene as a light yellow solid with a yield of 90%, m.p.96-97°C, 1 HNMR (CDCl 3 , 300MHz) δ: 7.40-7.57 (m, 4H), 7.99-8.21 (m, 2H), 8.26-8.32 (m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com