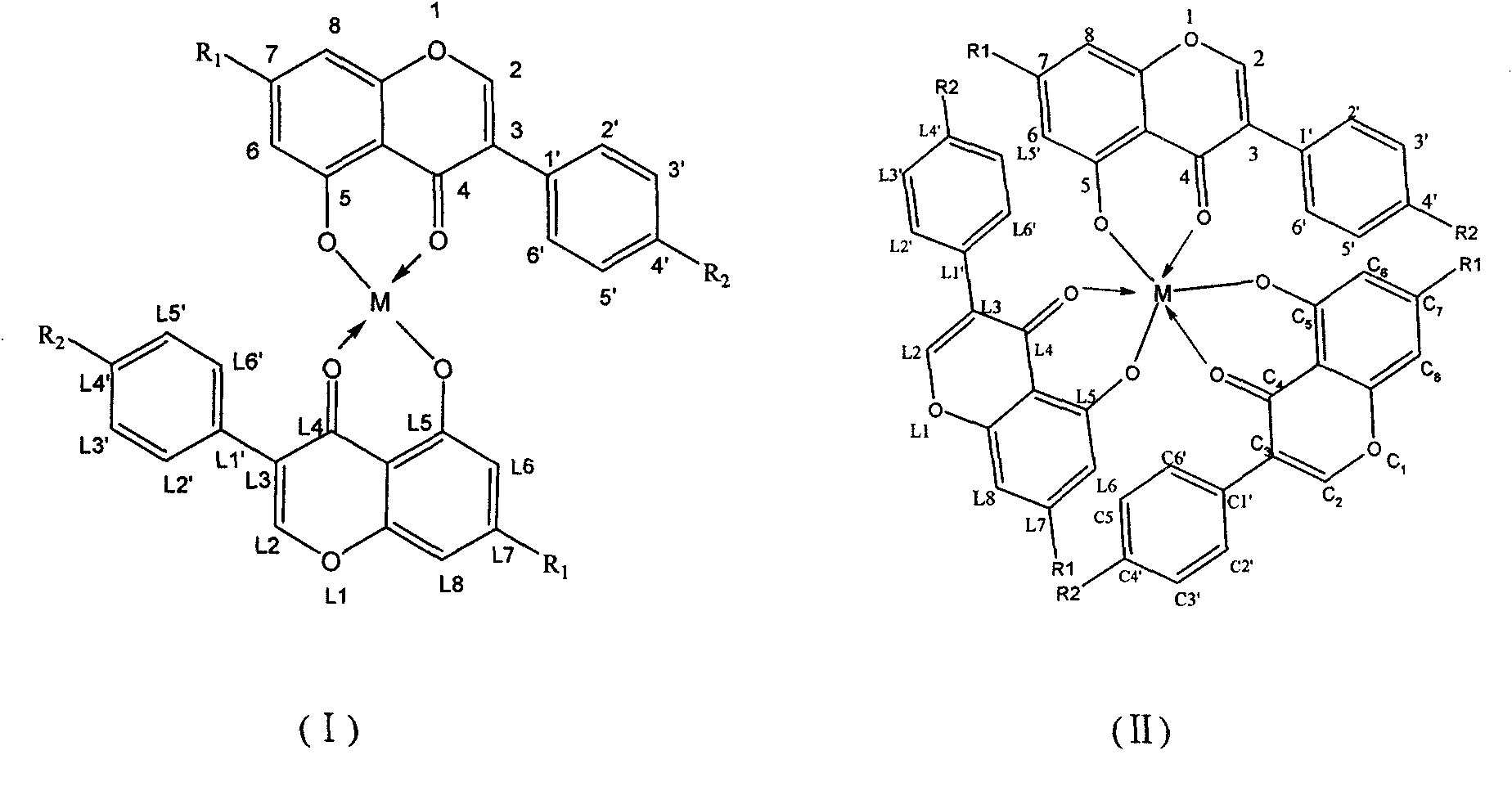
Method for preparing isoflavone metal complexes and anti-tumor medical application
A technology of metal complexes and hydroxy isoflavones, applied in the fields of natural medicinal chemistry, drug synthesis and pharmacology, can solve the problems of no isoflavone metal complex preparation methods and anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the preparation of 4'-methoxy-5,7-dihydroxyisoflavone-zinc complex (2)
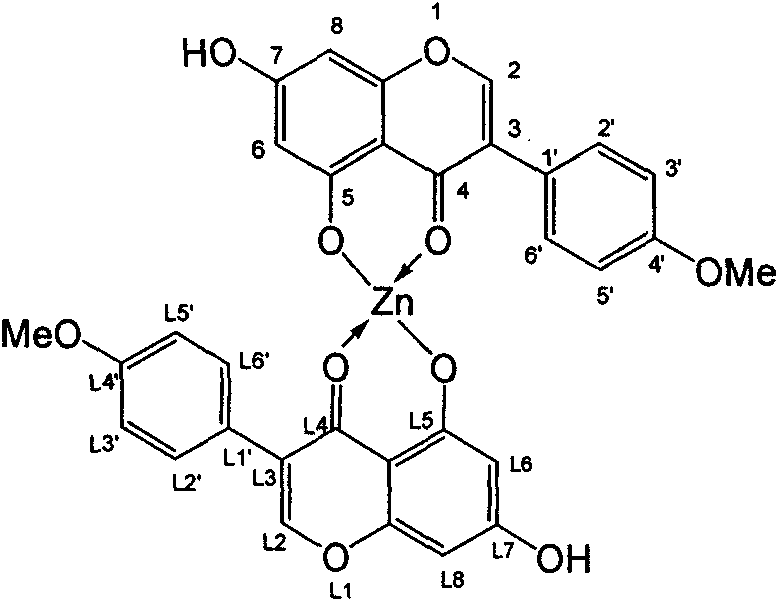
[0020] Weigh 0.284g of 4'-methoxy-5,7-dihydroxyisoflavone ligand into a flask, dissolve it with an appropriate amount of absolute ethanol, add triethylamine dropwise to adjust the pH to 7-8, and stir at 40°C 1h, centrifuge at 2000r / min for 5min, and absorb the supernatant. Also weigh Zn(OAc) 2 2H 2 O 0.22g, dissolved in absolute ethanol, added dropwise to the ligand solution and stirred continuously, stirred at 60°C until a precipitate appeared, then continued to react for 6h, filtered out the precipitate, washed several times with ethanol to obtain 0.25g Pale yellow 4'-methoxy-5,7-dihydroxyisoflavone-zinc complex with a yield of about 40.3%. Its structural formula is as follows (see specification attached) figure 2 ):
[0021] figure 2 4'-Methoxy-5,7-dihydroxyisoflavone-zinc complex
[0022] IR(KBr): 3369(O-H), 1645(C=O), 1611, 1578, 1514, 1411(C=C), 1280(C-O-H), 1187(C-O-C...
Embodiment 2
[0023] Embodiment 2: Preparation of 4'-methoxy-5,7-dihydroxyisoflavone-manganese complex (3)
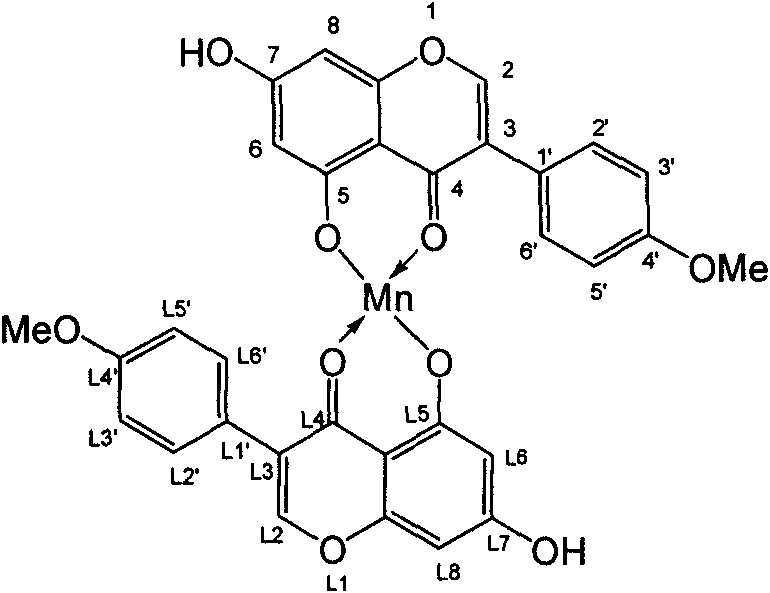
[0024] 4'-methoxyl group-5, the synthetic method step of 7-dihydroxyisoflavone-manganese complex is exactly the same as that of embodiment 1, just Zn(OAc) 2 2H 2 O was replaced by Mn(OAc) with the same mole number 2 2H 2 O, the brown solid was filtered out after the reaction, washed several times with ethanol, and the yield was about 42.1%. Its structural formula is as follows (see specification attached) image 3 ):
[0025] image 3 4'-Methoxy-5,7-dihydroxyisoflavone-manganese complex
[0026] IR(KBr): 3383(O-H), 1621(C=O), 1609, 1558, 1512, 1417(C=C), 1248(C-O-H), 1179(C-O-C), 732(Mn-O)cm -1 ; 1 HNMR (300MHz, DMSO-d 6 ): 3.71 (6H, s, 4', L4'-OCH 3 ), 5.32 (2H, s, 6, L6-H), 5.88 (2H, s, 8, L8-H), 6.49 (4H, d, 3′, 5′, L3′, L5′-H), 7.48 (4H, d, 2′, 6′, L2′, L6′-H), 8.34 (2H, s, 2, L2-H), 11.81 (2H, s, 7, L7-OH) ppm; EIMS m / z: 619.8 [M - , 100], 589.4, 336.1, 282.8; mol...
Embodiment 3
[0027] Embodiment 3: the preparation of 4'-methoxy-5,7-dihydroxyisoflavone-copper complex (4)
[0028] Weigh 0.284g of 4'-methoxy-5,7-dihydroxyisoflavone ligand into a flask, dissolve it with an appropriate amount of absolute ethanol, add triethylamine dropwise to adjust the pH to 7-8, and stir at 40°C 1h. Weigh another CuCl 2 2H 2O 0.168g was dissolved in absolute ethanol, and the ligand solution was added dropwise with continuous stirring. Immediately, solids were precipitated. Stirring was continued for 1 hour at 40°C, filtered, and the filter cake was discarded. The filtrate was placed at room temperature for several days to form a dark green precipitate. Suction filtration and washing several times with ethanol gave 0.5 g of powdery dark green solid with a yield of about 80.01%. Its structural formula is as follows (see specification attached) Figure 4 ):
[0029] Figure 4 4'-Methoxy-5,7-dihydroxyisoflavone-copper complex
[0030] IR(KBr): 3375(O-H), 1626(C=O), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com