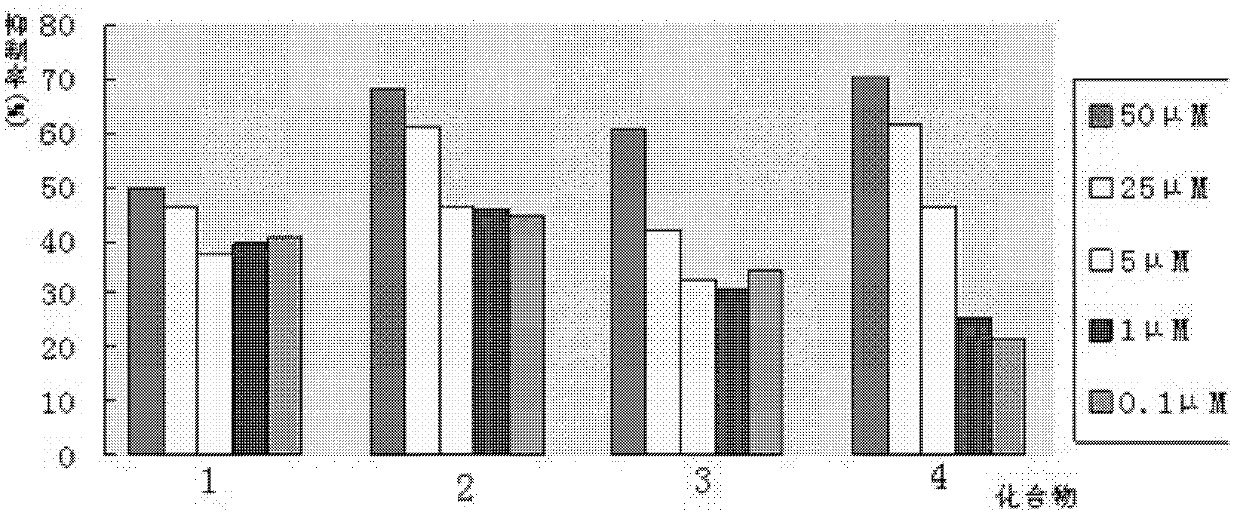
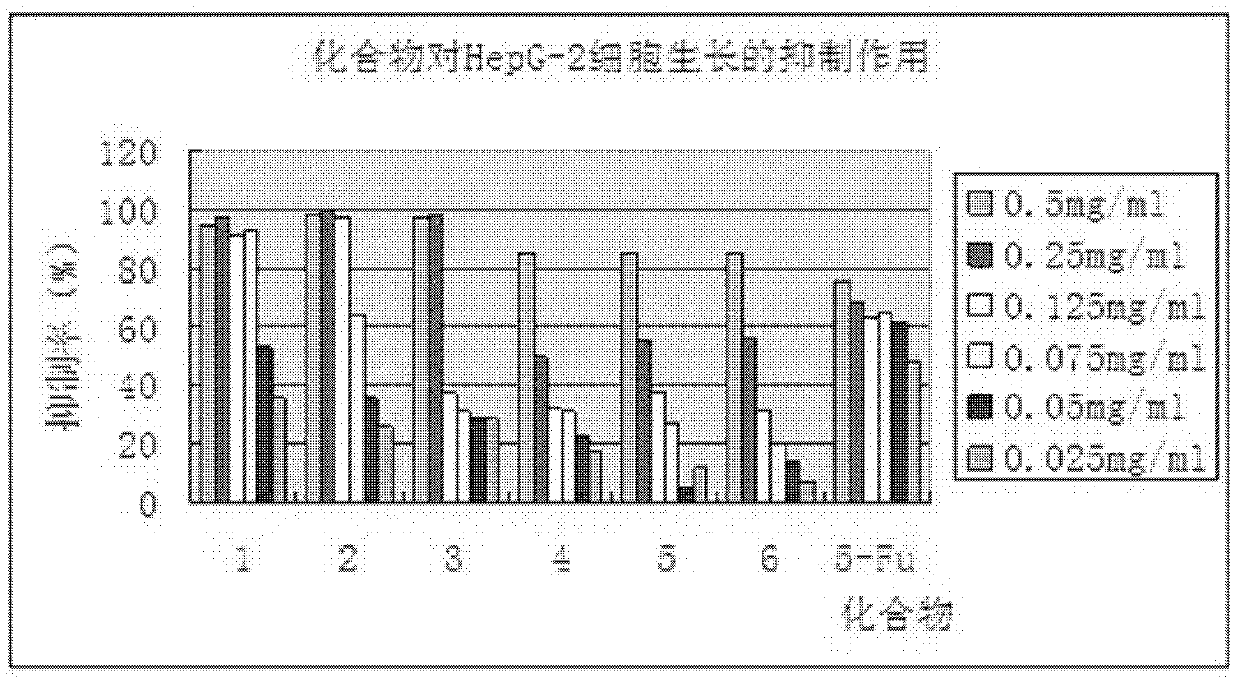
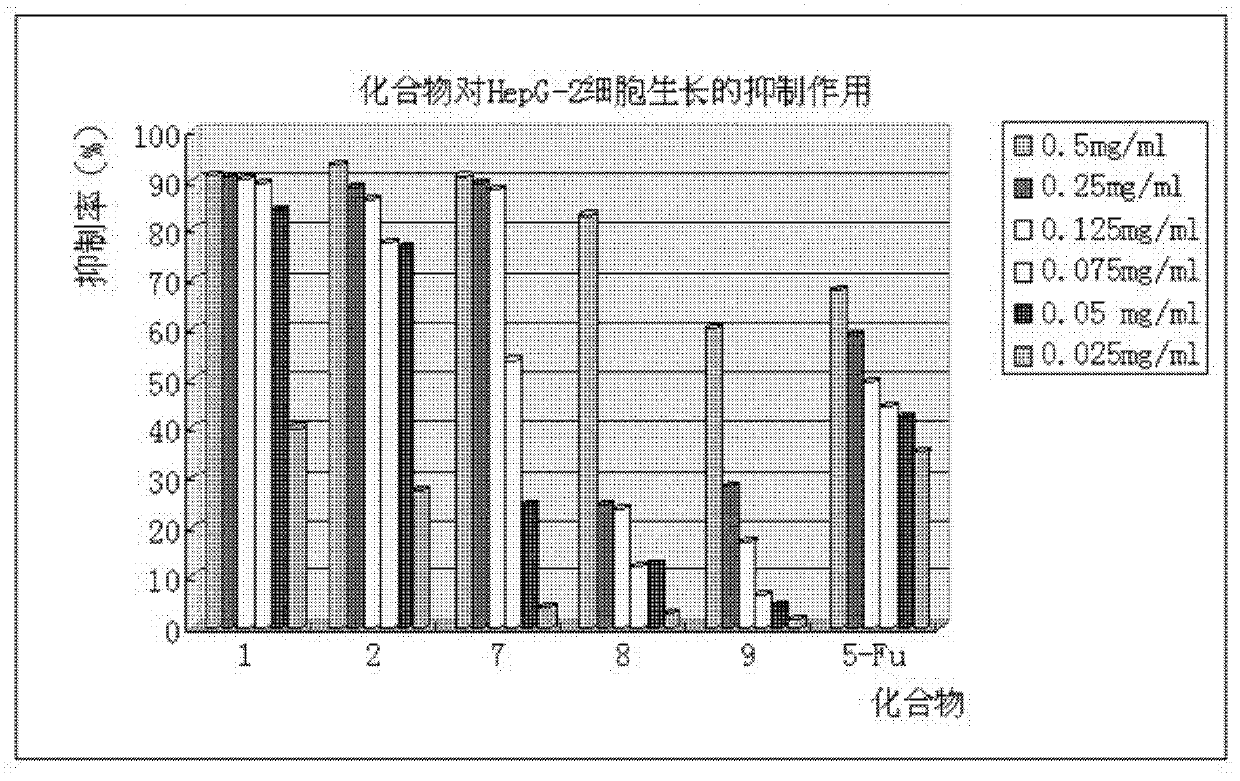
Phenylpiperazine derivatives for inhibiting tumor metastasis and tumor angiogenesis
A technology of phenylpiperazine and derivatives, which is applied in the field of medicine, can solve the problems of different mechanisms and degrees of tumor metastasis and spread, and achieves the effects of improved pharmacological activity, suitable molecular weight and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of intermediate 1-(4-methoxyphenyl)piperazine
[0053] Slowly add the mixture of diethanolamine diluted with chloroform dropwise under stirring to the mixed solution of thionyl chloride (the molar ratio of diethanolamine is 1:4) and chloroform cooled to about 0°C. After the dropwise addition, Continue to stir and react at room temperature for 2-5h, then gradually raise the temperature to 30-70°C, maintain this temperature for 2h, after the reaction is completed, add absolute ethanol, cool, filter under reduced pressure, wash the solid with ethanol and ether, and dry to obtain Bis(2-chloroethyl)amine hydrochloride, yield 97.8%, mp: 205.1-207.0°C.
[0054] Add 4.3g (24mmol) of bis(2-chloroethyl)amine hydrochloride and 2.5g (20mmol) of 4-methoxyaniline solid into 50mL of n-butanol, stir and mix evenly, irradiate with microwave for 6min under 195w power, and cool slightly Finally, add 1.3g (12mmol) of anhydrous sodium carbonate powder, continue microwave radiatio...
Embodiment 2
[0056] Synthesis of 3-Phenylcycloglutaric Anhydride
[0057] Mix 10.6g of benzaldehyde (0.1mol), 26.0mL (0.2mol) of ethyl acetoacetate, and 100mL of 95% ethanol, and slowly add 4.0mL of hexahydropyridine solution dropwise to the above mixture under stirring at room temperature, and stir for 1h to 3h . Filtrate under reduced pressure, wash the filter cake three times with absolute ethanol, recrystallize the resulting white precipitate with absolute ethanol, and dry in vacuo to obtain 32.6 g of white needle crystals, namely 2,4-diacetyl-3-phenylpentadiene Acid diethyl ester, the yield is 93.5%, and the melting point is 155.1-156.9°C.
[0058] Add 32.6g (0.094mol) diethyl 2,4-diacetyl-3-phenylglutarate in batches to 100mL 50% KOH solution under stirring, and control the temperature between 30-80°C for 2 hours. Stop heating, cool to room temperature, and adjust the reaction solution with concentrated hydrochloric acid in an ice-water bath until the pH is approximately equal to 2...
Embodiment 3
[0065] Synthesis of 5-oxo-3-(4-methoxyphenyl)-5-(4-tolylpiperazine)pentanoic acid (No. JA3031)
[0066]Add 1.0g (5mmol) 1-(4-methylphenyl) piperazine hydrochloride, 1.1g (5mmol) 3-(4-methoxyphenyl) cyclopentanedioic anhydride, 0.5 mL of pyridine, 15mL of absolute ethanol, microwave radiation reaction under 130w power for 25-30min, TLC monitoring until the reaction is complete (petroleum ether: ethyl acetate: methanol: glacial acetic acid = 0.5mL: 0.5mL: 1 drop: 1 drop), while Filtrate hot, rinse with absolute ethanol, and the obtained filtrate stands overnight for crystallization. Suction filtration under reduced pressure, and the resulting filter cake was rinsed with a small amount of absolute ethanol. Recrystallized from absolute ethanol and dried in vacuo to obtain 1.5 g of a colorless needle crystal, yield 65.2%, mp: 157.4-159.4°C. UV(λ / nm): λmax=247; HPLC(min): Rt=6.990; IR(KBr, σ / cm-1): 3064~2510(m, ν O-H , νAr-HB), 3029 (s, νasCH 3 ), 2006 (s, νasCH 2 ), 2911(s, νa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com