Anti-tumor glutaminase inhibitor, tumor angiogenesis inhibitor drug compound and application thereof
A tumor angiogenesis and glutaminase technology, applied in the field of biomedicine, can solve the problems of high cost and insignificant effect, and achieve the effect of inhibiting rapid growth, significant synergy and synergy, and significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] [Example 1] Preparation of glutaminase inhibitor compound GI-003 (glutaminase inhibitor-003)
[0096] This example provides a preparation method for glutaminase inhibitor GI-003, the specific steps are:
[0097] (1) Equimolar thiosemicarbazide and ethoxyacetic acid were refluxed at 90° C. for 3 hours under the catalysis of phosphorus oxychloride. The reaction mixture was cooled to room temperature, ice water was added, and the pH was adjusted to 14 with sodium hydroxide. The white precipitate was washed with water. Vacuum dry. It is an intermediate product [1]
[0098]
[0099] 5-(ethoxymethyl)-1,3,4-thiadiazol-2-amine
[0100] (5-(ethoxymethyl)-1,3,4-thiadiazol-2-amine)
[0101] (2) Mix the intermediate product [1] with equal moles of phenylacetyl chloride and pyridine, heat until the mixture is uniform, cool the solution to room temperature, add methanol and filter, leaving a thick solid, which is the intermediate product [2]
[0102]
[0103] N-(5-(hydrox...
Embodiment 2
[0113] [Example 2]: Preparation of glutaminase inhibitor compound GI-006 (glutaminase inhibitor-006)
[0114] This example provides a preparation method for glutaminase inhibitor GI-006, the specific steps are:
[0115] (1) Equimolar thiosemicarbazide and ethoxyacetic acid were refluxed at 90° C. for 3 hours under the catalysis of phosphorus oxychloride. The reaction mixture was cooled to room temperature, ice water was added, and the pH was adjusted to 14 with sodium hydroxide. The white precipitate was washed with water. Vacuum dry. It is an intermediate product [1]
[0116]
[0117] 5-(ethoxymethyl)-1,3,4-thiadiazol-2-amine
[0118] (5-(ethoxymethyl)-1,3,4-thiadiazol-2-amine)
[0119] (2) Mix the intermediate product [1] with equal moles of phenylacetyl chloride and pyridine, heat until the mixture is uniform, cool the solution to room temperature, add methanol and filter, leaving a thick solid, which is the intermediate product [2]
[0120]
[0121] N-(5-(hydro...
Embodiment 3
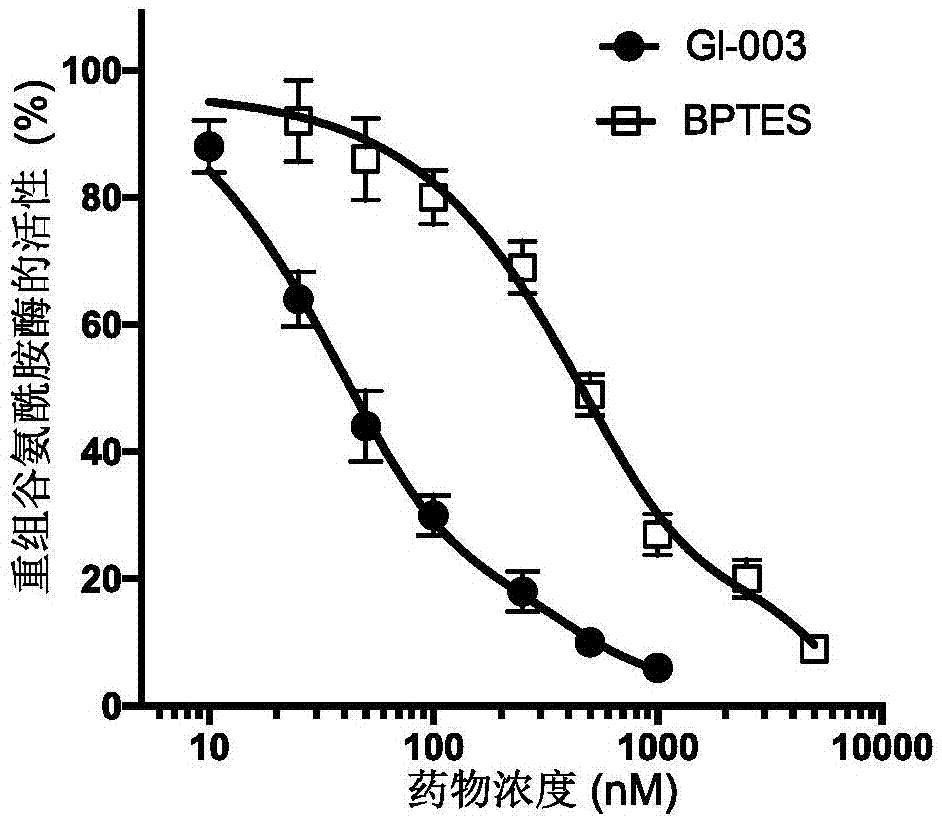
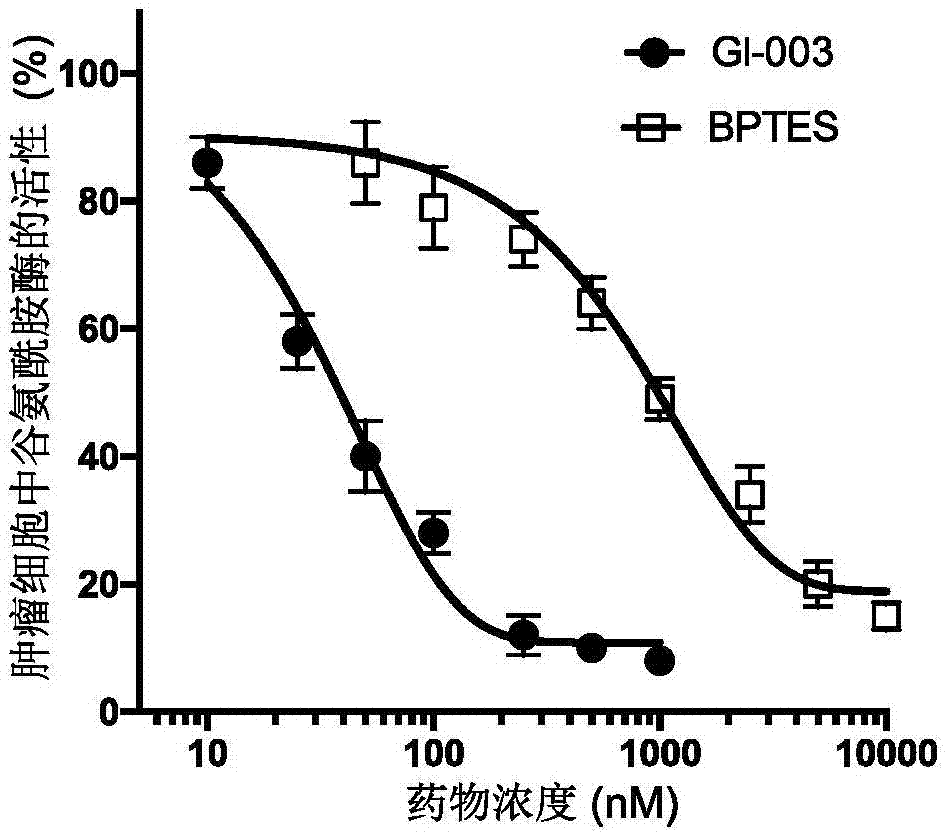
[0135] [Example 3] Compound GI-003 inhibits the activity of recombinant glutaminase
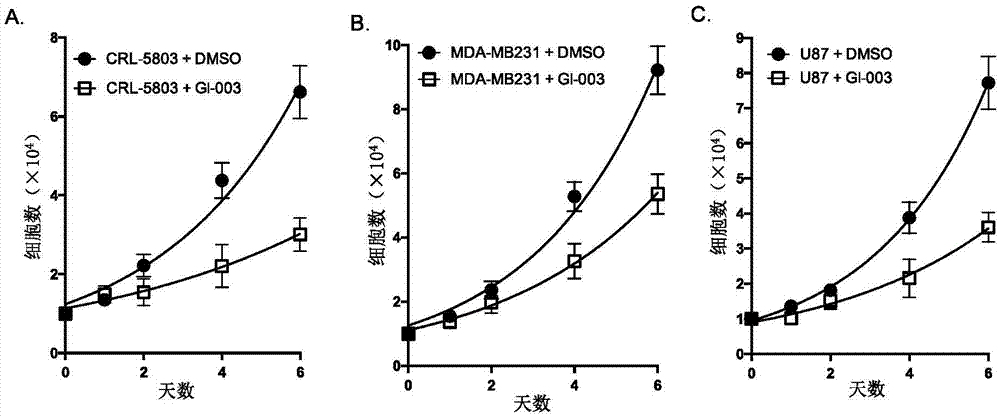
[0136] The recombinant protein of mouse glutaminase (molecular weight: 66kD) was expressed and prepared in Escherichia coli, and its enzymatic activity was measured after being treated with different concentrations of compound GI-003 or BPTES. The specific steps are as follows: mouse glutaminase was expressed in Escherichia coli using the pET28a plasmid (Novagen: catalog number: 69864-3) cloned with the gene of mouse glutaminase with histidine attached to the N-terminal. The recombinant glutaminase protein is further purified by anion exchange column chromatography. 1 μM glutaminase recombinant protein was mixed with different concentrations of compound GI-003 or the known glutaminase inhibitor BPTES— Warm up to a final volume of 80 μl and spin for 30 minutes. Compound GI-003 or BPTES was diluted with DMSO and the added volume was kept constant (5 μl) in different reactions. Glutamine was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com