4-aromatic aminoquinazoline derivative and purpose thereof
A kind of technology of aromatic aminoquinazolines and derivatives, which can be applied in the field of medicinal chemistry and can solve the problems of undiscovered transformation and the like
Inactive Publication Date: 2010-10-13
SOUTHEAST UNIV
View PDF3 Cites 17 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
Login to View More
Abstract
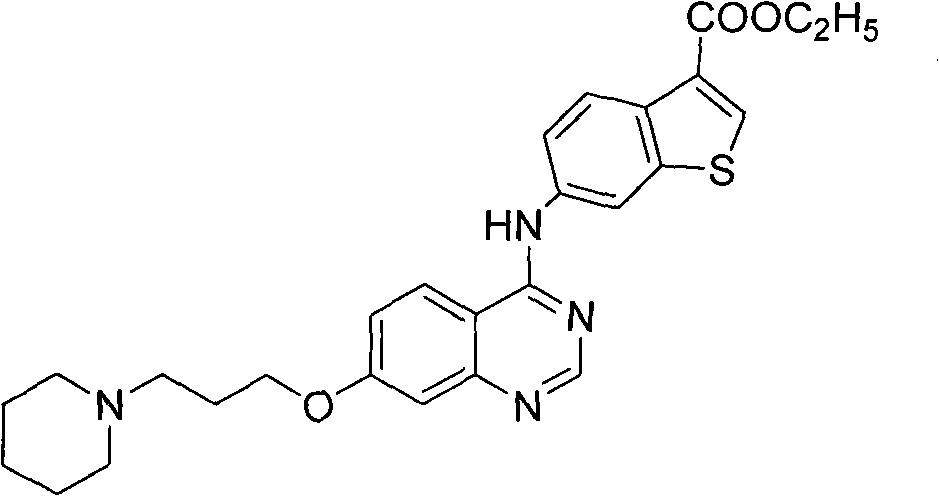
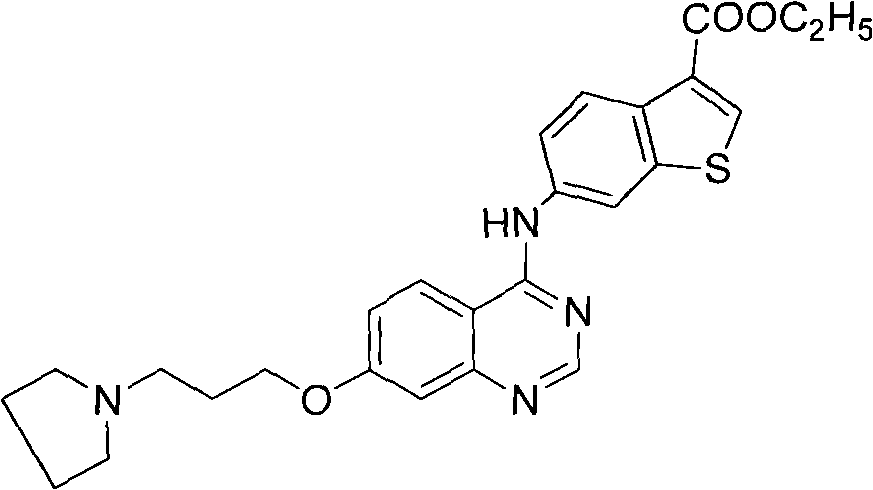
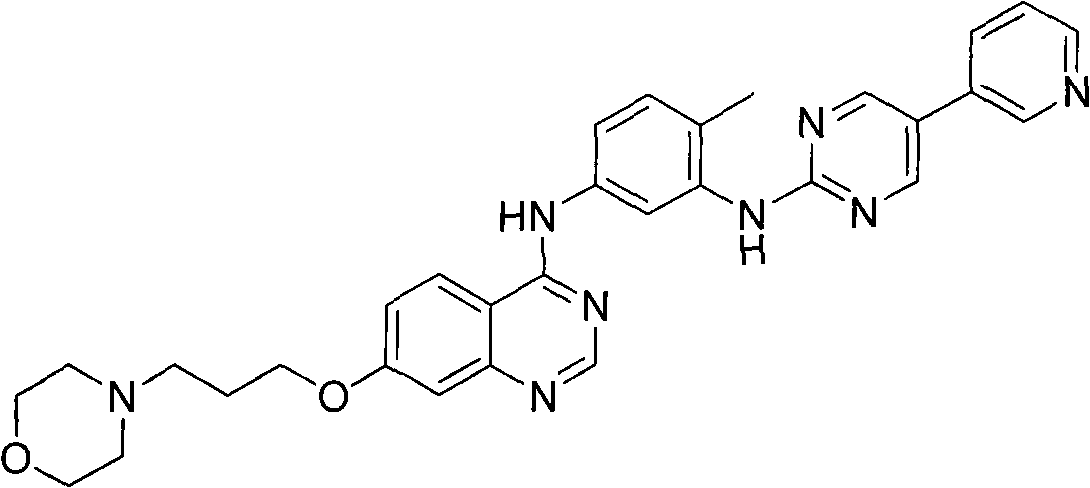
The invention discloses a 4-aromatic aminoquinazoline derivative and a purpose thereof, wherein the 4-aromatic aminoquinazoline derivative has a structure shown as the formula (I). Pharmacological experiments indicate that the compounds or the pharmaceutically acceptable salts thereof can restrain the multiplication of multiple tumor cells. In the formula, ring A is a benzene ring, an alkylbenzene ring, a benzothiophene ring or an alkyl benzothiophene ring, R1 is hydrogen or alkoxy, n is an integer selected from 1 to 6, R2 is -NR4R5. R4 or R5 is respectively selected from hydrogen, alkyl and naphthenic base, or R4 and R5 are combined to form a substituted or unsubstituted heterolipid ring group, wherein the substituted group is an alkyl or hydroxyalkyl. R3 is an ester group, a substituted or unsubstituted aniline, substituted or unsubstituted pyridine amino, substituted or unsubstituted pyrimidine amino, substituted or unsubstituted benzyl carbamido, substituted or unsubstituted picolyl carbamido, substituted or unsubstituted pyrimidine methyl carbamido or substituted or unsubstituted naphthenic carbamido, wherein the substituted group is halogen, alkyl, phenyl or pyridyl or pyrimidyl, and preferably, the substituted group is phenyl, pyridyl or pyrimidyl.
Description
Technical field The invention belongs to the field of medicinal chemistry, and specifically relates to an Aurora kinase inhibitor, that is, a 4-arylaminoquinazoline derivative and its use. Background technique Protein Kinases (Protein Kinases) include protein serine / threonine kinase (STK) and protein tyrosine kinase (Protein Tyrosine Kinase, PTK). Among them, a new family of serine / threonine kinases involved in mitosis, the Aurora family, has been gradually recognized. They are divided into three categories: Aurora-A, Aurora-B and Aurora-C, which mainly regulate the functions of centrosomes and microtubules. Ensure the correct separation of the centrosome and the complete division of the cytoplasm. The function of Aurora kinase involves various important events of mitosis, including centrioles, spindle changes, chromosome segregation and cytokinesis. In normal cells, Aurora kinase relies on various forms of regulatory means to express and activate in specific time and space, ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D401/14C07D409/12C07D239/94C07D401/12A61K31/5377A61K31/517A61P35/00A61P35/02
Inventor 吉民郑友广李丽丽郑明
Owner SOUTHEAST UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com