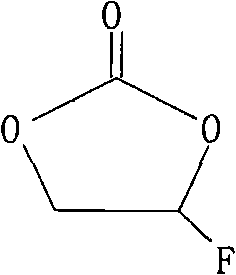
Method for synthesizing fluoroethylene carbonate by phase-transfer catalysis
A technology of fluoroethylene carbonate and chloroethylene carbonate, which is applied in the field of phase-transfer catalytic synthesis of fluoroethylene carbonate, and can solve problems such as difficult-to-control reactions, safety accidents, and unsuitability for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 245 grams of chloroethylene carbonate to a dry 1000 milliliter three-necked flask, add 400 milliliters of butanone, 170 grams of potassium fluoride, and 1 gram of 18-crown-6 at the same time, react by heating, and react for 4 hours at reflux temperature. After finishing, cooling, filtering, filtrate gas chromatographic analysis, analysis result shows that 80.5% chloroethylene carbonate reacts, and the selectivity of fluoroethylene carbonate is 85.4%.
Embodiment 2
[0023] Add 245 grams of chloroethylene carbonate to a dry 1000 milliliter three-necked flask, add 350 milliliters of butanone, 170 grams of potassium fluoride, and 1.5 grams of 18-crown-6 at the same time, heat the reaction, and react at 80 ° C for 7 hours. After finishing, cooling, filtering, gas chromatographic analysis of the filtrate, analysis result shows that 85.3% of chloroethylene carbonate reacts, and the selectivity of fluoroethylene carbonate is 82.7%.
Embodiment 3
[0025] Add 375 g of chloroethylene carbonate to a dry 1500 ml three-necked flask, simultaneously add 350 ml of acetonitrile, 250 g of potassium fluoride, and 2.0 g of 18-crown-6, heat the reaction, and reflux at 90° C. for 5 hours. After the reaction was finished, it was cooled, filtered, and the filtrate was analyzed by gas chromatography. The analysis results showed that 82.6% of chloroethylene carbonate had reacted, and the selectivity of fluoroethylene carbonate was 85.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com